Sorry my mistake.
I was talking about the vaccine ZyCov… today’s news also says it can hit Indian markets by June
Instead of launching own vaccine which they can continue as side job, Can they import technology/Process from Bharat Biotech and start manufacturing COVAXIN.
Question is do they have necessary infra required for it. As per my understanding CADILA has BSL 1 Lab while COVAXIN needs BSL 3. So How complex is the up gradation Can they adapt to the ocassion ?
BSL: Bio Safety Level/Lab
Disc: Invested in CADILA around 622
Upgrading to BSL-3 will require multiple steps 1. Infra 2. Training 3. Certification … all this certainly will take good time.
… I am not an expert just based on layman’ thinking + what I am reading around.
Company launches Trastuzumab Emtansine for breast cancer treatment in India at a 80% discount to innovator prices.
Disclosure: Invested (position size here)
This is good development for breast cancer patient.
The cost from innovator Roche / via his subsidiary Gennentech is more than 2 Lakhs (160 mg) and 1.35 Lakhs (100 mg) post discount.
Here are my notes from today’s concall:
- Faced pricing challenges in US, flu season was non-existent
- Launched pegfilgrastim in Russia (only player to do this)
- Q4 domestic growth is from base business and not covid products. 60:40 split in mass:specialty. Specialty business has done significantly better than market
- US focus will be on orphan drugs, target segments are CNS, pain and gastrointestinal
- 2 biosimilar products for developed markets but this is post FY25
- Transdermal + hormonal products are filed out of Moraiya. See 4 critical launches after resolution of Moraiya
- Vaccine: Have secured device supply chain, will be assembled in India. Expect to start with 1cr./month and scale it to 2.5-3 cr./month. Also looking for 1-2 contract manufacturers for additional capacity. Being tested in ~1000 children, they were approved because its needle free
- COVID product portfolio is at lower gross margins compared to base business (conscious company strategy to make covid drugs more affordable)
- Can maintain R&D at 8% of sales over the next few years
- Want base EBITDA margins to be ~22%
Disclosure: Invested (position size here)
Cadila introduces a cool way to verify if the drug is real or a counterfeit by using a scratch code and verifying it on their application. Also, a useful way to collect consumer data.
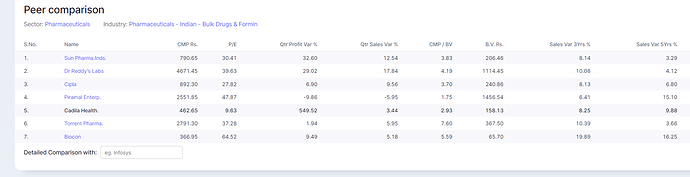
How much of total sales is of Saroglitazar Magnesium?
Gets approval for ZycovD from DCGI
Disclosure: Not invested
AR21 notes:
- US business:
o Maintained top 3 ranking in 60% of product families
o Launched complex injectables such as Fondaparinux and Doxorubicin Liposomal injection in last couple of years. Expects to launch one more complex injectable soon
o Filed 22 ANDAs; received 35 ANDA approvals (11 tentative); launched 30 new products, filed 9 DMFs;
o Filed 1 NDA, 1 pre-NDA (pNDA) and 5 pre-Investigational New Drug (pIND) requests with the USFDA. Plans to file 1 more NDA in pain management in FY22.
o In-licensed 20 complex generics till date (with 2 exclusive first to file)
o Acquired rights to Copper Histidinate product candidate CUTX-101 from Cyprium Therapeutics Inc (orphan drug with fast track designation for Menkes disease). Filing should be completed by December 2021 and expect earliest case approval in Q1CY22
o Regulatory:
Warning letter Moraiya: Completed remediation and submitted final update to FDA in November 2020
Site transferred for two injectable products (Succinylcholine Chloride injection, Doxycycline injection) from Moraiya to Liva site and re-introduced them in the market - Indian business:
o Branded formulations (i.e. business excluding generics) business 10% growth
o
o Commercialized AmphoTLC™ (Amphotericin B Liposome for Injection 50mg), the first & only complex generic with bioequivalence to Gilead’s AmBisome, to treat Mucormycosis or Black Fungus (in-licensed from TLC Taiwan)
o 14 brands feature among top 300 with9 brands having sales of >100 cr.
- Consumer wellness:
o Nutralite: Lockdown impacted performance severely in the institutional segment. However, the brand witnessed sequential recovery month by month and has now reached pre-Covid levels during the last quarter. Launched Nutralite DoodhShakti Probiotic Butter Spread and Nutralite DoodhShakti Pure Ghee in January 2021, thus entering dairy category.
o SugarFree and Nycil brands witnessed strong double digit growth
o Redeemed NCDs worth 1500 cr.
o E-Commerce grew by more >250% accounting for ~3.6% of domestic revenue
o Glucon-D: Brand got adversely impacted by COVID-19 challenges, cyclone Amphan and early monsoon in some parts of the country. Launched Glucon-D ImmunoVolt
o Complan grew in high single digits - Emerging market:
o Launched 5 products in Brazil. Brazil business grew in double digit on back of revival in demand for branded generics while in Mexico, growth remained robust with a more focused approach in CNS segment
o Achieved highest ever market share of 7.31% in Sri Lanka with 24 brands ranked number one in their respective molecule categories - Animal health:
o Sold for 2’921 cr.
o Sales of Rs. 599.8 cr.
o Launched 6 new products and received 3 new marketing authorizations for exports business
o Working on new growth avenues in regulated markets of US and Europe - COVID products:
o Fabidac (Favipiravir), Iveloc (Ivermectin), Derinide, Depotex, Zincee (Vit-C tablet), Cimune (combination of Vit-C (Ascorbic Acid) and Elemental Zinc in India.
o Launched ‘Virafin’ (Pegylated Interferon alpha-2b) for Treating Moderate Covid Patients
o Its Remdesivir (Remdac) was cheapest among Indian competitors
o Ramped up hydroxychloroquine supply from 9 to 30 tons/month
o Supplied 6.36 cr. injections and 3.01 cr. ampules of dexamethasone across India - Innovation
o Saroglitazar Magnesium (India): Received approved from DCGI for treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) thus becoming first medicine for treatment of NAFLD in India.
o Saroglitazar Magnesium (USA): Successfully completed Phase II clinical trials for Primary Biliary Cholangitis (PBC) indication. USFDA granted Orphan Drug Designation (ODD) and Fast Track Designation to Saroglitazar Magnesium in the treatment of patients with PBC
o Saroglitazar Magnesium (USA): Received USFDA approval to initiate Phase II(b) clinical trials of for NASH and F2/F3Fibrosis in the US
o Completed Phase I clinical trials for an anti-malarial drug candidate which is being developed to provide a single-dose cure for malaria
o Completed patients recruitment for Phase III trials in India for another Investigational New Drug (IND), Desidustat, for treating anemia both in dialysis and non-dialysis dependent CKD patients
o Desidustat is being pursued in the US for Chemotherapy Induced Anaemia (CIA). At present, the clinical trials are going on in the US for this indication
o Received DCGI approval to initiate Phase I trials of ZYIL1, a novel oral small molecule targeted at selectively suppressing inflammation caused by NLRP3 inflammasome - Vaccines
o Developed world’s first Plasmid DNA vaccine (also works for age groups of 12-18 years). The DNA platform used is a plug and play technology and can be easily and quickly adapted to virus mutations. Applied using PharmaJet® needle free system, Tropis®, leading to significant reduction in side effect. Stored at 2-8 C. Buidling up capacity to manufacture 1 cr. doses per month
o Received marketing authorization for Hep-B vaccine (rDNA) and Pentavalent Vaccine in India
o Successfully completed Phase II/ III trials in adults and adolescents in India for Tetanus Diphtheria (Td) vaccine and submitted the marketing authorization application to DCGI - Biologics
o Successfully completed Phase III clinical trials in India for Rituximab.
o Launched Pegfilgrastim in Russia (first ever launch in Russia). Opens door for fast track approval in other CIS countries
o Commercialized biosimilars in 9 emerging market countries - Invests 7-8% of sales in R&D (1400 research scientists)
- Debt reduced to 3’496.3 cr. from 6’740 cr. in FY20
- Has 36 manufacturing plants globally
- Number of employees: 24’412, Average increase in employee salaries other than managerial personnel was 10.48%, average increase in managerial remuneration was 12.26%
- Number of shareholders: 227’976, price (low): 258.6, price (high): 509.35
Disclosure: Not invested
The fortunes of Cadila are likely to change dramatically with the launch of its vaccine ZyCov-D. This has been approved for age of 12 and above. As schools and colleges reopen, parents would like their kids to be vaccinated. Thus the demand in below 18 segment, where this is the only Covid vaccine, could be huge.
Has anyone worked on the likely impact of the vaccine in the fundamentals of the company over the next two years? This could be very interesting
Zycov-D is needle free, unlike Covaxin but is a 3 dose vaccine. Will it make a difference?
not sure wat u mentioned will happen. Mkts have already factored the other income & hence the optically low PE. The PE wont re-rate on this one-time gain. I haven’t studied wat they’ll do with the proceeds bt they will either go to treasury or given as one-off dividend probly.
The PE ex-proceeds of Animal care biz is already 15+ which is pretty much the mkt case for all US based players due to margin/de-growth issues.
Disclosure :- not invested.
@kartiks :-
I read what you posted & got ur point. not sure why u deleted it. Technically, yes I concede wat u say might hold - as m total novice in technicals. Bt my point was that the PE wont adjust fundamentally to one-off earnings as the markets are aware of it. And the stock is already selling at a reasonable multiple excl. the one-off earnings.