Wow, excellent news indeed! This approval is transformational for the company and shareholders.
Discl - hold and adding since 2013
Wow, excellent news indeed! This approval is transformational for the company and shareholders.
Discl - hold and adding since 2013
Quarter to Quarter Updates (Q3FY21 to Q4FY21)
Hyderabad, India, February 26th, 2021
Natco Pharma Limited (NSE: NATCOPHARM; BSE: 524816) is pleased to announce the launch of Brivaracetam tablets under brand BRECITA in India. Brivaracetam is indicated towards treatment of epilepsy. Brivaracetam is developed by UCB Pharma and currently marketed in India by Dr Reddy’s under brand name Briviact®. NATCO’s BRECITA tablets will be available in two strengths of 50mg and 100mg at significantly lower MRP prices of INR 25/- and INR 35/- per tablet respectively. Epilepsy patients in India is estimated to be between 5-10 million, as per GEMIND guidelines.
Hyderabad, India, March 3rd, 2021
Natco Pharma Limited (NSE: NATCOPHARM; BSE: 524816) is pleased to announce that its marketing partner, Breckenridge Pharmaceutical Inc. (BPI), has received final approval for its Abbreviated New Drug Application (ANDA) for Everolimus Tablets (generic for Afinitor®) from the U.S. Food and Drug Administration (USFDA).
NATCO’s partner BPI plans to launch 2.5 mg, 5 mg and 7.5 mg strengths of the product shortly within the next few weeks. The launch of lOmg strength of the product is subject to confidential terms of a settlement and license agreement entered into with the owner of the Afinitor® brand. The launch date of lOmg strength of the product will be announced at a later date. The above strengths of Everolimus are indicated in the treatment of breast cancer and a few other types of cancers. As per industry sales data, Afinitor® and its therapeutic equivalents had generated annual sales of $712 million in USA during the twelve months ending December 2020.
Hyderabad, India, April 10th , 2021
NATCO Pharma Limited (NSE: NATCOPHARM; BSE: 524816) is pleased to announce that its marketing and distribution partner Alvogen Pine Brook LLC, has received tentative approval for our Abbreviated New Drug Application (ANDA) for Ibrutinib Tablets 560mg, 420mg, 280mg and 140mg strengths (generic
for IMBRUVICA® ), from the U.S. Food and Drug Administration (USFDA). Based on our ANDA filing date and the approval timeline, NATCO believes that we are eligible for 180 days of sole generic marketing exclusivity for all the strengths of the tablet dosage form of the product at the time of launch. As per industry sales data, IMBRUVICA® (Tablet and Capsule dosage forms) had generated annual sales of $3.7 billion during the twelve months period ending December 2020 in the US market, of this, all the strengths of IMBRUVICA® Tablets alone generated sales of $3.0 billion during the same period.
Hyderabad, India, April 26th, 2021 - NATCO seeks Emergency approval of Molnupiravir capsules for Covid-19 treatment
Hyderabad, India, May 3rd, 2021 - NATCO receives Emergency Use approval for Baricitinib tablets for Covid-19 treatment
Hyderabad, India, May 17, 2021 - NATCO signs Voluntary Licensing Agreement with Lilly for Baricitinib for Covid-19 in India
NATCO starts Phase-III Clinical Trial of Molnupiravir capsules for Covid-19 treatment Hyderabad, India, May 21st, 2021
May 22nd, 2021
The above strengths of Everolimus are indicated in the Prophylaxis of Organ Rejection in Kidney Transplantation and Liver Transplantation. As per industry sales data,
ZORTRESS® and its therapeutic equivalents had generated annual sales of $162million during the twelve months ending March 2021 in the US
Hyderabad, India, May 22nd, 2021
Agro Chemicals
Hyderabad, India, February 5th, 2021
Hyderabad, India, March 15th, 2021
I guess Laurus is the API supplier for Lenalidomidems-laurus-labs-pvt-ltd-wc-1597806687.pdf (5.1 MB)
Note : This doesn’t mean that Laurus is the supplier, we need find if Laurus has FDA approval to supply this key API , request others to lookup and share.
Laurus cannot be the API supplier for US sales because they don’t have an approved DMF. In an ANDA application, companies have to mention an approved API supplier which can be verified in the DMF file. Now we can see that Laurus is not in the approved list of API suppliers for lenalidomide (Q1CY21 DMF file). These are the currently approved list of API suppliers
| 23410 | A | II | 12/29/2009 | MYLAN LABORATORIES LTD | LENALIDOMIDE |
|---|---|---|---|---|---|
| 24264 | A | II | 12/2/2010 | MYLAN API US LLC | LENALIDOMIDE |
| 25872 | I | II | 3/30/2012 | DR REDDYS LABORATORIES LTD | LENALIDOMIDE |
| 27116 | A | II | 6/4/2013 | CADILA HEALTHCARE LTD | LENALIDOMIDE |
| 27905 | A | II | 2/3/2014 | RELIANCE LIFE SCIENCES PVT LTD | LENALIDOMIDE |
| 29628 | A | II | 9/16/2016 | CIPLA LTD | LENALIDOMIDE |
| 31549 | A | II | 12/27/2017 | SUN PHARMACEUTICAL INDUSTRIES LTD | LENALIDOMIDE |
| 31590 | A | II | 3/28/2017 | MSN LABORATORIES PRIVATE LTD | LENALIDOMIDE |
| 31804 | A | II | 6/2/2017 | CHANGZHOU PHARMACEUTICAL FACTORY | LENALIDOMIDE |
| 33084 | A | II | 4/4/2020 | SHILPA MEDICARE LTD | LENALIDOMIDE |
| 34724 | A | II | 3/27/2020 | SYNTHON BV | LENALIDOMIDE |
| 34956 | A | II | 7/31/2020 | BIOCON LTD | LENALIDOMIDE |
| 35505 | A | II | 12/31/2020 | DR REDDYS LABORATORIES LTD | LENALIDOMIDE |
| 32279 | A | II | 12/1/2017 | HETERO LABS LTD | LENALIDOMIDE (FORM-H1) |
| 34650 | A | II | 2/28/2020 | ALEMBIC PHARMACEUTICALS LTD | LENALIDOMIDE (FORM-H1) |
| 29691 | A | II | 9/30/2015 | DR REDDYS LABORATORIES LTD | LENALIDOMIDE (POVIDONE PREMIX) |
| 31960 | A | II | 9/13/2017 | MSN LABORATORIES PRIVATE LTD | LENALIDOMIDE [ROUTE CODE LW] |
| 29690 | A | II | 9/30/2015 | DR REDDYS LABORATORIES LTD | LENALIDOMIDE DIMETHYLFORMAMIDE SOLVATE |
| 35556 | A | II | 2/1/2021 | CIPLA LTD | LENALIDOMIDE HEMIHYDRATE |
| 30795 | A | V | 8/31/2016 | CELGENE CORP | LENALIDOMIDE REMS HOSTED SYSTEM |
Also, here is the list of APIs which Laurus is approved for.
| DMF# | STATUS | TYPE | SUBMIT DATE | HOLDER | SUBJECT |
|---|---|---|---|---|---|
| 33031 | A | II | 8/30/2018 | LAURUS LABS LTD | ABACAVIR SULFATE USP (PROCESS-2) |
| 28818 | A | II | 12/15/2014 | LAURUS LABS LTD | ABACAVIR SULFATE, USP |
| 30934 | A | II | 9/29/2016 | LAURUS LABS LTD | ATAZANAVIR SULFATE |
| 32214 | A | II | 7/18/2018 | LAURUS LABS LTD | ATORVASTATIN CALCIUM TRIHYDRATE USP |
| 26846 | A | II | 2/7/2013 | LAURUS LABS LTD | AZACITIDINE |
| 25903 | A | II | 3/19/2012 | LAURUS LABS LTD | BIMATOPROST |
| 26774 | A | II | 12/19/2012 | LAURUS LABS LTD | BORTEZOMIB |
| 22510 | A | II | 1/28/2009 | LAURUS LABS LTD | BRIMONIDINE TARTRATE |
| 28293 | A | II | 6/12/2014 | LAURUS LABS LTD | CABAZITAXEL |
| 31233 | A | II | 1/22/2017 | LAURUS LABS LTD | CANAGLIFLOZIN |
| 21918 | A | II | 8/29/2008 | LAURUS LABS LTD | CARBOPLATIN USP DRUG SUBSTANCE |
| 30115 | A | II | 3/22/2016 | LAURUS LABS LTD | CARFILZOMIB |
| 21917 | A | II | 8/29/2008 | LAURUS LABS LTD | CISPLATIN USP DRUG SUBSTANCE |
| 31198 | A | II | 10/24/2017 | LAURUS LABS LTD | DARUNAVIR |
| 23138 | A | II | 9/24/2009 | LAURUS LABS LTD | DOCETAXEL ANHYDROUS USP |
| 26595 | A | II | 10/30/2012 | LAURUS LABS LTD | DOCETAXEL ANHYDROUS, USP (PROCESS-2) |
| 30935 | A | II | 9/28/2016 | LAURUS LABS LTD | DOLUTEGRAVIR SODIUM |
| 31855 | A | II | 7/5/2017 | LAURUS LABS LTD | DOLUTEGRAVIR SODIUM (PROCESS-2) |
| 33768 | A | II | 4/23/2019 | LAURUS LABS LTD | DOLUTEGRAVIR SODIUM (PROCESS-3) |
| 21964 | A | II | 9/10/2008 | LAURUS LABS LTD | EFAVIRENZ USP |
| 26242 | A | II | 7/17/2012 | LAURUS LABS LTD | EFAVIRENZ USP (PROCESS-2) |
| 32881 | A | II | 6/26/2018 | LAURUS LABS LTD | EMPAGLIFLOZIN |
| 23773 | A | II | 4/28/2010 | LAURUS LABS LTD | EMTRICITABINE |
| 31196 | A | II | 12/30/2016 | LAURUS LABS LTD | ENZALUTAMIDE |
| 34313 | A | II | 1/8/2020 | LAURUS LABS LTD | ERLOTINIB HYDROCHLORIDE |
| 22800 | A | II | 5/16/2009 | LAURUS LABS LTD | GEMCITABINE HYDROCHLORIDE USP |
| 31956 | A | II | 9/8/2017 | LAURUS LABS LTD | HYDROXYCHLOROQUINE SULFATE USP |
| 27089 | A | II | 5/9/2013 | LAURUS LABS LTD | IMATINIB MESYLATE |
| 33312 | A | II | 11/16/2018 | LAURUS LABS LTD | IMATINIB MESYLATE (PROCESS-2) |
| 22296 | A | II | 11/21/2008 | LAURUS LABS LTD | IRINOTECAN HYDROCHLORIDE TRIHYDRATE USP |
| 31854 | A | II | 7/7/2017 | LAURUS LABS LTD | LAMIVUDINE USP |
| 32633 | A | II | 3/31/2018 | LAURUS LABS LTD | LAMIVUDINE USP (PROCESS 1) |
| 23539 | A | II | 2/13/2010 | LAURUS LABS LTD | LATANOPROST USP |
| 33919 | A | II | 6/25/2019 | LAURUS LABS LTD | LOPINAVIR USP |
| 31957 | A | II | 9/15/2017 | LAURUS LABS LTD | MACITENTAN |
| 30114 | A | II | 5/4/2016 | LAURUS LABS LTD | METFORMIN HYDROCHLORIDE USP |
| 23736 | A | II | 4/16/2010 | LAURUS LABS LTD | MONTELUKAST SODIUM USP |
| 33767 | A | II | 1/10/2020 | LAURUS LABS LTD | OSELTAMIVIR PHOSPHATE USP |
| 22387 | A | II | 12/22/2008 | LAURUS LABS LTD | OXALIPLATIN USP |
| 26431 | A | II | 9/11/2012 | LAURUS LABS LTD | PEMETREXED DISODIUM |
| 32739 | A | II | 5/4/2018 | LAURUS LABS LTD | PIRFENIDONE |
| 31415 | A | II | 5/6/2017 | LAURUS LABS LTD | PREGABALIN |
| 29841 | A | II | 12/8/2015 | LAURUS LABS LTD | RILPIVIRINE HYDROCHLORIDE |
| 33342 | A | II | 8/30/2019 | LAURUS LABS LTD | RITONAVIR USP |
| 33647 | A | II | 6/7/2019 | LAURUS LABS LTD | SACUBITRIL VALSARTAN 3NA COMPLEX |
| 35584 | A | II | 3/16/2021 | LAURUS LABS LTD | SITAGLIPTIN PHOSPHATE MONOHYDRATE USP |
| 32068 | A | II | 11/10/2017 | LAURUS LABS LTD | SOFOSBUVIR |
| 33541 | A | II | 2/28/2019 | LAURUS LABS LTD | TENOFOVIR ALAFENAMIDE FUMARATE |
| 23450 | I | II | 1/7/2010 | LAURUS LABS LTD | TENOFOVIR DISOPROXIL FUMARATE |
| 27087 | A | II | 5/9/2013 | LAURUS LABS LTD | TENOFOVIR DISOPROXIL FUMARATE (PROCESS-2) |
| 31958 | A | II | 10/17/2017 | LAURUS LABS LTD | TENOFOVIR DISOPROXIL FUMARATE (PROCESS-3) |
| 27967 | A | II | 3/5/2014 | LAURUS LABS LTD | THALIDOMIDE USP |
Hi,
Thanks for the price info. Have read a lot about Natco in this thread and am really convinced about the future prospects of the company. Have just few doubts which are not really something very material but just wanted to understand out of curiosity. Why has the total FII stakeholding in last 4 years gone down by 10% almost on a frequent quarter on quarter basis. Well the upside is that DII’s have been increasing stake by almost same 10% in last 4 years. But there’s been hardly any overall price gain in those 4 years. Not sure what to make up for it 
Also, isn’t CMP/Sales ratio of almost above 10 is a bit on the higher side, unless ofcourse the mkts are expecting Topline to double in 3 years max. Opinions from more experienced in this company will be really appreciated. Thanks 
Hi Harsh,
I checked the link/atachment provided by @Rafi_Syed for Laurus labs API, and in that there’s a export list of 10 API’s where they do mention LenaliDomide as approval for exports. However, I also read your comment about the US filings in which Laurus Labs is not mentioned. Not sure if they might be listed next quarter. From what I understand from Sajal Kapoors webinar sometime back that the API supplier for this would be a big beneficiary. Not sure if it would be Biocon or Laurus that he was referring to.
it is shilpa medicare
https://vbshilpa.com/api-products.php
You can see here that Shilpa has USDMF for Lenalidomide
Dr Reddy, Cipla, Hetero, Shilpa, Sun Pharma, Alembic, Reliance Life science, Biocon, MSN Laboratories also have the usdmf filing, that does not mean that they are supplying to NATCO. You can check here
I was asking is there any information specifically that shilpa is supplying to Natco?
Its Laurus Labs : Why am saying this?
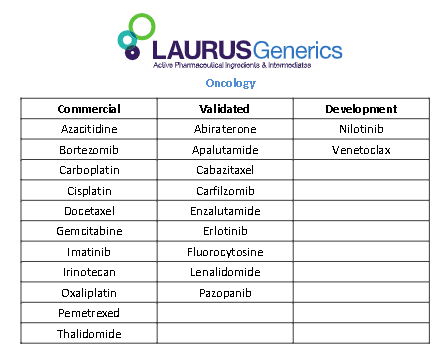
Links : Presentation | Laurus Labs | PDF | PharmaCompass.com
Validated : API EU Written Confirmation | Abacavir Sulfate (USP/IH/Ph. Eur/Ph. Int) | M/s. Laurus Labs Pvt. Ltd., | Written Confirmation | PharmaCompass.com
As Laurus already has a good relation with NATCO and main of the times Chava has said that they are partnering with NATCO for HEP-C (I know HEP-C is not related here however the partnership is openly discussed by both parties)
Laurus has not filed DMF for this API. Last month this was asked during the call
Nimish Mehta: Finally, a word on the API opportunity, Lenalidomide now that is likely to be US-centric market
next year?
Dr. Satyanarayana Chava: We do not have any DMF in US. So, we cannot comment on that right now.
U.S. lawmakers took aim at drugmaker Celgene for continually boosting the price of the cancer drug Revlimid at the start of a two-day hearing on Wednesday, saying in some cases the moves were designed to help company executives meet bonus targets.
Senator grilling https://youtu.be/_U9sbwuhMBo
Feel free to flag if you feel not relevant
Longterm technical chart - cup and handle, new all time high
Discl - biased, last transaction buy at 1100
I dont know why would anyone flag this post with long term price chart
Natco has received ANDA approval for Carfilzomib Vials.
As per industry sales data, KYPROLIS® had generated annual sales of $696million during the
twelve months ending December 2020 in the US. 10mg strength alone generated sales of
$63million for the same period
Coming back to this question, if we read the ANDA approval document for NATCO (link), we will find that the DMF file specified in Natco’s ANDA application is “030795”.
If we go to the latest DMF file and search for “30795” this is the result.
| DMF# | STATUS | TYPE | SUBMIT DATE | HOLDER | SUBJECT |
|---|---|---|---|---|---|
| 30795 | A | V | 8/31/2016 | CELGENE CORP | LENALIDOMIDE REMS HOSTED SYSTEM |
If we go to the lenalidomide REMS website (https://www.lenalidomiderems.com/), this is what we find.
Does this mean the API is being sourced from celgene? Or does this mean we will only know once generic API supplies of lenalidomide is started (August 16, 2021)?
REMS has nothing to do with API
REMS - Risk Evaluation and Mitigation Strategy. Only a few drugs are in this category - lenalidomide being one of them.
Lenalidomide is teratogenic - if this drug is taken by a pregnant female, it causes limb anomalies in the child born (phocomelia is the medical term for such limb malformations). Babies are born with very short arms and legs. This happened in 1960s when thalidomide was given to pregnant females as an antinausea drug - babies were born with very short limbs and the drug was banned . It made a comeback in the 1990s in the treatment of myeloma. Lenalidomide is the better version of thalidomide but has the same teratogenic potential.
In view of the above risk, doctors have to educate the patient very well before prescribing/dispensing. Physicians have to mention all the potential side effects including its teratogenic potential. Patients taking this drug should not become pregnant or should not impregnate their partner if male. This has to be documented online - they call it REMS in the USA. This is a legal document which makes it clear the precautions the patient has to follow whilst on this therapy. The patients are not allowed to distribute this drug to other people because of the above risk…
The REMS system used to have the name Revlimid (Celgene’s lenalidomide brand name) on their website - they are changing the name to lenalidomide (chemical name) since there will be several companies in 2022 who are allowed to sell it in the US. Celgene is still in charge of the REMS for lenalidomide - they have to follow up on serious side effects as it occurs as part of the monitoring system of REMS.
Nothing major in my view…
I am pretty sure lenalidomide API is manufactured within the company…
How is this possible given that Natco doesn’t have a DMF filed for this?
I attended the con call, my rough notes
Covid was the reason for the sub-optimal financial performance this year - oncology/cardio/hep C business in India were significantly affected. Oncology price controls also had an adverse impact.
Covid also affected Tamiflu sale in the USA - there was virtually no flu in the USA because of covid related isolation
Covid related drug are expected to do well in the near to medium term. Domestic business is better in Q1 than last quarter
There are launching pheromones in agri business - not clear on details
USA
Copaxone is going good.
Lanthanum is stable
Doxil - reduced market share
Tamiflu - virtually nothing
Everolimus should do well
Revlimid 30% profit share - not clear on price details. Single digit market share in 2022
Canada has done well. Brazil still loss making.
Domestic business will recover post covid
Rajeev N has not given any guidance for this year - because of covid
Last year he had given a guidance of 20% growth but covid changed everything…
Discl - holding and adding since 2013. Last buy transaction 1084 rs
I am not 100% sure…
I thought one ought to have the ability to manufacture API to challenge a patent in the court…
Here are my notes from their concall
2 gaps in current portfolio: US frontend (valuations are not demanding, looking for an acquisition), India branded (valuations are crazy)
Everolimus should be a good product
Crop: Launching a pesticide for management of pink bollworm in cotton crop (pheromone based mating disruption technology)
Higher inventory because of:
o Agri inventory cycle requires inventory buildup in March quarter to service demand in June quarter
o COVID specific pharma portfolio requires high inventory buildup
Domestic oncology portfolio has witnessed higher competition and pricing control from government along with covid specific demand glut (lower number of chemo therapies where Natco is dominant) – this is very high margin business
Targeting 7-8 filings/year over the next couple of years
Very bad flu season impacted US sales
Will not guide because market is very uncertain, Q1FY22 will be better than Q4FY21
Revlimid launch will be in March 2022, high single digit market share and exclusivity for certain time period along with 1/3rd profit share (1/3rd to Natco, rest to Teva)
Disclosure: Invested