If everybody is agreeing and guranteed a volume there is no incentive to quote a discounted rate .
Hi,
NATCO PHARMA LTD - TO ACQUIRE 0.96% STAKE IN NATCO PHARMA (CANADA) INC.
What is their current stake in Canada unit & How will this affect the sharehloders of Natco pharma?
Thanks,
Deb
NATCO receives tentative approval for
tablets (generic for IMBRUVICA ®
)
ANDA in the US market
can anybody share where is Natco gonna source the revilmid api from???I understand it will not make it in-house.
There are about 12 approved DMFs for lenalidomide
Natco Seeks emergency approval to launch Molnupiravir for Covid-19 in India - The Economic Times - https://m.economictimes.com/industry/healthcare/biotech/pharmaceuticals/natco-seeks-emergency-approval-to-launch-molnupiravir-for-covid-19-in-india/articleshow/82256562.cms?utm_source=whatsapp_pwa&utm_medium=social&utm_campaign=socialsharebuttons
Is there a source where we can check whether they have approached the dcgi with any data backed study …such info might help in determining the possible launch date with revenue is sight …
Regards
Divyansh
1619626515093_11981_2021.pdf (422.6 KB) does this oŕder mean natco can sell ctpr. Legal heads please explain the order.
Hi All,
Sharing my notes from Natco’s latest credit rating
Hope this helps…!
Regards,
Yogansh Jeswani
Disclosure: Invested
Ref: PR/ 3 /2021-22 Press Release
NATCOreceives Emergency Use approval for Baricitinib
tablets for Covid-19 treatment
Hyderabad, India, May 3rd, 2021
Natco Pharma Limited (NSE: NATCOPHARM; BSE: 524816) has received Emergency
Use approval for Baricitinib tablets, 1mg, 2mg and 4mg strengths from Central
Drugs Standard Control Organization (CDSCO) in India. Baricitinib in combination
with Remdesivir, is used for treatment of COVID-19 positive patients.
Natco will be requesting a Compulsory License based on emergency use and in light
of the grave and serious public health emergency across India due to the Pandemic.
The company is ready to launch the product this week, so as to make the product
available to suffering patients across India.
Baricitinib is a chemical compound and is an azetidine and cyclobutane derivative. The drug was officially approved for the treatment of rheumatoid arthritis. It acts as an inhibitor for Janus Kinase (JAK) blocking the sub-types JAK1 and JAK2, the former being a human tyrosine kinase protein essential for signalling certain type I and type II cytokine and the latter is a non-receptor tyrosine kinase and is also involved in signalling by the members of type II cytokine and many other receptors. The cytokines contribute to the induction and maintenance of inflammation and the drug Baricitinib disrupts their activation mechanism.
In early 2020, with the help of AI, Baricitinib was identified as a drug which could block the Covid-19 virus infection process. Simulation studies predicted that Baricitinib could reduce the ability of the virus to infect the lungs in case of a person afflicted with Covid-19 infection. Anecdotal studies reported that a combination of Baricitinib and Remdesivir is more effective in the treatment of Covid -19 infection than Remdesivir alone. This was particularly true of the patients receiving high flow oxygen or non-invasive ventilation. It was also concluded that the combination drug of Baricitinib and Remdesivir is associated with fewer adverse effects, reduced recovery time and accelerated improvement in the clinical status of the patients. In November 2020, US FDA approved the combination of Baricitinib and Remdesivir for the treatment of Covid -19 infection. Natco referred to these developments and US FDA approval in its application for the grant of a compulsory license. Natco also observed that Eli Lilly and Company, licensee of the patent holder, imported 9000 tablets in 2019 and 2020 and sold the drug under the brand name of Olumiant at a retail price of Indian Rupees 3230 per tablet.
Natco in its compulsory license application proposed to sell Baricitinib tablet at a retail price of Indian Rupees 15-30 and pay 7% royalty to the patent owner.
Eli Lilly News Release - Innovator granted access to use this molecule Royalty free - Source
Reuters reports only Cipla, Lupin and Sun are working in collaboration with Eli Lilly , I think Natco is the first one to ask for their permission and there is no response given to them (as per the earlier money control article )
I was looking for the API suppliers from India and found four have have this molecule (correct me if my understanding is wrong ) but looks like none of them have the finished API product, either they are under development or still in initial phase. Since NATCO is first one to use emergency act that means they have all the necessary API KSM to produce this drug.
Let us hope this will help the much needed medical supplies to the needy also it will open up the options for affordable treatment.
Hi,
Thank you for sharing the article.Was wondering how much sales/revenue it can garner for Natco?
As the details say that the Baricitinib is used along with Remedesiver(Which is having a lot of demand) for treatment of Covid-19 patients.
Thanks,
Deb
18% CAGR in Sales over 5 years is excellent with ROCE ~ 14%, with healthy OPM% and NPM%, this is an interesting stock.
- Almost debt-free and dividend paying
What worries me is
- this is almost 8 times price to sales
- 0.5 times capital turnover ratio, that drags the ROCE
- Currently trading at a P/E of 35, it has even traded at P/E of 11 in the past. So if the stock gets de-rated, there can be a bear hammering. Even 5 year and 7 year P/E are at 20
Lenalidomide approval is expected anytime. The market is pricing in the future at 35 PE.

A simple thread and quick Refresher about Natco from JST Investments
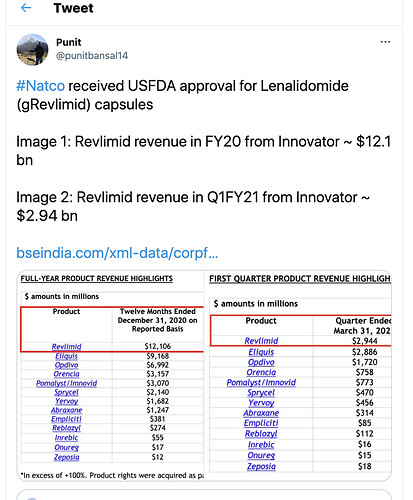
Looks like the wait is over for REVLIMID approval, which Natco received today, as per filing.
Latest market size as per this twitt (screenshot below)
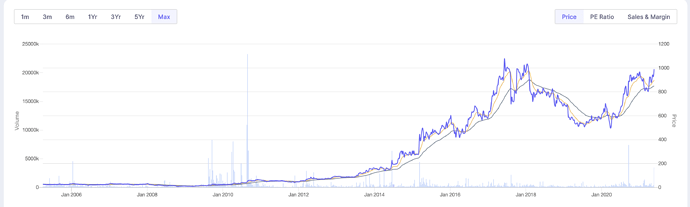
The stock is setup nicely on a multi year view.
Once it cross 1077 level, which it did set on June 2017, it has high chances of going into higher orbit.