FY23 annual report notes
Long term aim
- Become global leader in the parenteral domain with a portfolio including nanoparticles, liposomes, microspheres, suspensions, and emulsions
- Invest in vaccine platform, innovative aesthetic and neurological solutions, and novel immunotherapies for cancer treatment
New product development, launches & R&D
- Launched 20 generic products in FY23 with 10 more to be commercialized soon
- 22 new products are in development phase
- Received DCGI approval for manufacturing and marketing of Biapenem Dual Chamber IV Bag
- Launched Ceftazidime + Avibactam – received good response
- Launched Dydrogesterone - was very successful. Working on Sustained Release Tablet form of Dydrogesterone
- Launched new Zinc based multivitamin & multimineral formulation to augment mass market division
- Will launch novel Oral form of Isavuconazole for treatment of invasive aspergillosis
- Will launch novel analgesic - Polmacoxib (Stellar division) for osteoarthritic pain. Also plan to launch combinations of Polmacoxib with a muscle relaxant
- Will launch moisturizing agents, anti-aging, Hyperpigmentation, Sunscreen agents in Aesthaderm
- Will launch a novel once-a-week anti-infective – Dalbavancin for 1st time in India (received marketing authorization from DCGI)
- Initiated registration for a range of Hyaluronic Acid based Dermal fillers to complement and augment product basket in aesthaderm
- Received approval for Anaesthetic product Prilocaine for supply to China
- On verge of a break-through in development of an innovative topical formulation of Botulinum Toxin which will be launched very soon
- Initiated trial of a new product made from an Indian gum resin by a standardized extraction process for use asthma management. Clinical trial will be completed in FY24
- Looking to expand market penetration for Enoxaparin within the infertility segment
- Imported technology: Penem in dual chamber and super purified menotropin
- R&D: 7.3 cr. (vs 7.1 cr. in FY22). Capital expenditure of 2.1 cr. (vs 2.6 cr. in FY22) & revenue expenditure of 5.2 cr. (vs 4.4 cr. in FY22)
- Installed high-capacity lyophilizers to increase batch sizes, reduce processing times in lyophilization cycles and extend shelf life of products
- Lyophilized products span therapeutic areas, including Antibiotics, Antifungals, Cardiac, Infertility treatments, Antiviral solutions and proton-pump inhibitors (PPIs)
Company developments
- Maintained leadership position in lyophilized injectable products in Anti-fungal and Anti-bacterial segments
- Hospital reach: 1500+, field force: 1000+
- Surveyed 8000+ hospitals and started supplies in few of these
- Regained business in infertility segment that was lost during COVID-19. Focus is on building brands within Hormonal category (HMG, HCG including most potent form of HMG which reduces chance of failures of IVF cycles) and offer newer drugs and newer drug delivery systems with PFS (Pre-filled Syringes) and DCS (Dual Chamber Syringes)
- Stunnox gained momentum to reach amongst top 2 brands
- Completed a split face clinical trial between Stunnox and Botox
- Gufic’s brand of Boswellia Serrata continues to retain leadership through brand Sallaki
- Arisia completed training of 50+ Gynaecologists in cosmetic gynaecology using toxin, fillers and FDA approved energy based devices. Arisia also trained 20+ Dermatologists and 10+ Neurologists in advanced techniques of using botulinum toxin injections in various aesthetic and neurological indications
- Auto-loading and unloading technology for lyophilized products ensured no human intervention during manufacturing process at Navsari
- APIs will largely be used captively
Capex (187.6 cr. vs 87.45 cr. in FY22)
- Validation of Indore facility will be completed by H2FY24
- Operational launch of Indore facility is scheduled in October 2023, with revenue contribution from Q3FY24
- Capital advances: 55.87 cr. (vs 34.32 cr. in FY22)
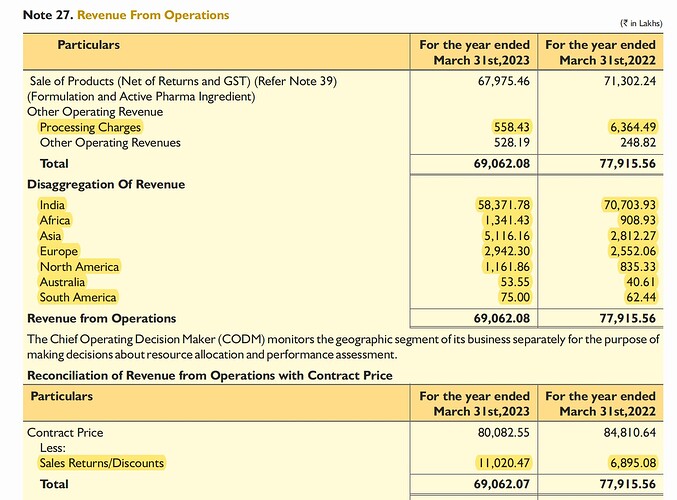
Geographical segment & financials
-
Exports to 20+ countries, 190+ products with 150+ products under pipeline in 40+ countries
-
Received 4 new international approvals from Columbia, Uganda and Ecuador
-
Key markets: India, Germany, Switzerland, South Africa, Russia, Canada, Brazil, Europe
-
Sales returns: 110.2 cr. (vs 68.95 cr. in FY22)
-
Sales return provision: 6.4 cr. (vs 7.6 cr. in FY22)
-
Geographical breakup
-
No Single Customer accounted for 10%+ of revenues in FY22 and FY23
Forex
- Income: 86 cr. (vs 73.2 cr. in FY22)
- Expense: 271.6 cr. (vs 230 cr. in FY22)
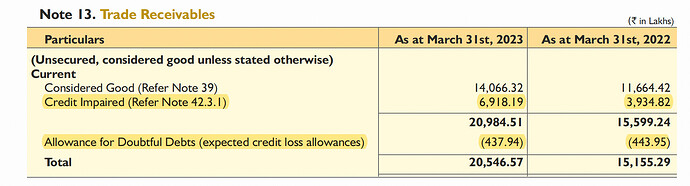
Trade receivables (huge red flag): impairment of 69 cr. (vs 39 cr. in FY22)
Credit risk increase: In FY23, they had 10 customers (same as FY22) that owed them 100 cr. (vs 111 cr. in FY22) and accounted for 48.71 % and 73.25 % respectively
Miscellaneous
- Employees: 1628 (vs 1382 in FY22)
- Average percentile increase in remuneration for employees other than managerial personnel was 12.32%, increase in managerial remuneration was 1.99%
- ESOP 2023 program: authorized 5 lakh stock options
- Very high employee churn (40% turnover in permanent employees)
- Equity investment of 77.68 lakhs in Selvax Pty (13.07 lakh shares ~ 2.67% equity)
- Share price: 177.1 (low), 290 (high)
- Shareholders: 39’563
- Auditor remuneration: 36.93 lakhs (vs 32.8 lakhs in FY22)
- Number of customer complaints: 23 (vs 38 in FY22)
- Is in the process of implementing SAP to enhance and fortify internal control mechanism
- Bankers: The Saraswat Co-operative Bank, UCO Bank, SBI, Axis, HDFC Bank. Bank loan rates was ~8% (except 11.4% for a 4 cr. property loan from others)
General trends
-
India’s drug pricing authority sanctioned 12.1% price increase for scheduled drugs
-
Over 60% of APIs are procured from foreign sources, with certain APIs exhibiting import dependency rates of 80% to 90%
-
500 Indian API manufacturers contributing about 8% in global API Industry
-
India supplies 20% of generics globally
-
Indian Pharma Industry had growth of 7% in 2022 with market size of Rs. 1.94 cr. Chronic therapies grew by 9% and acute therapies by 5%
-
IPM has grown at a CAGR of ~11% in domestic and ~16% in exports over last 2 decades
Disclosure: Invested (position size here, no transactions in last-30 days)