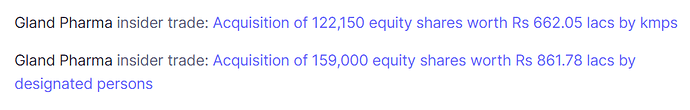Gland Pharma has the capability of making the vaccine. What I understood they are going to announce it shortly.
Disc: invetsed
Gland Pharma doesnot manufacture any vaccines. They are going to do only filling of vaccines in vial(packaging job). Margin will be very less
MOSL CoverageGLAND20210217MOSLICPG044-2.pdf (7.1 MB)
Gland Pharma will be leveraging its manufacturing capabilities for the production of Sputnik V COVID-19 vaccine.
The agreement will see Gland Pharma utilising its Drug Substance and Drug Product facilities at its sites in Hyderabad.
The production is expected to commence from third quarter of 2021 for estimated delivery starting from fourth quarter
of 2021.
Under the terms of the agreement, Gland Pharma will first undertake technology transfer of the drug substance to its
manufacturing facilities. After successful technology transfer, Gland Pharma will then undertake manufacturing of drug
substance and drug product filling into vials under aseptic conditions. Gland Pharma’s expertise in manufacturing of
sterile injectable at significant scale will support in establishing a stable supply of COVID-19 vaccine.
BSE announcement
Happy to see company is not doing fill finished only but entering drug substance also (probably better margin)
Thanks
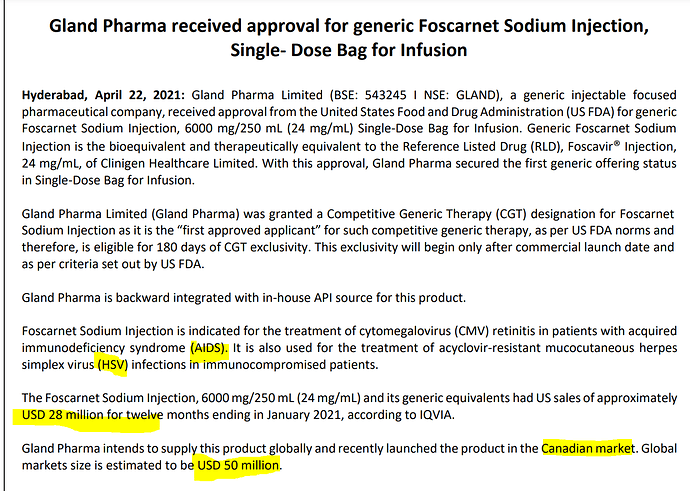
Gland Pharma received approval for generic Foscarnet Sodium Injection,
Single- Dose Bag for Infusion
good nos gland
invested since ipo n listing
Con-call Audio Time Stamp : 6:52
Concall Transcript
One new development ( I tried to lookup for this acquisition of Vitane Biolgics) Gland Pharma acquired Vitane Biologics (based in Genome Valley, Hyderabad), there is no intimation about this to the exchange, is this just happened recently ?
Gland Pharma Limited, Q4 2021 Earnings Call, May 17, 2021.pdf (336.7 KB)
Summary
-
Year-on-year growth of 40% in revenue
-
Emerging Markets
196% YOY Growth
Entered new markets like Singapore, Israel, Saudi Arabia and CIS countries through new partners during this period ( All these opportunities arised due to COVID situaion, got quick approvals) -
China 6 products that Fosun has said which are at various stages of getting an approval, I think 3 under Q3 revenue and the others are yet to start Q3 revenue. (Revenue wise not very significant to start with )
-
Biosimilar opportunity
Will be used this company assests in vaccine manufacturing
Started this journey with acquistion of Vitane Biologics
Our approach is alwasy to start with contract manfacturing development, this will help us to get the processes into our development environment and then we build from there for adjacencies and optionalities once the project is over
Many equiries are coming in on Biosimilar manufacturing so this acquistion will help to cater those needs
Vaccine contract we have in hand is manfacturing of the substance and then fill and finish -
Exploring other M&A opportunities that will help build capabilities to strengthen products and technology infrastructure, such as long-acting injectables, steroidal hormonal products, suspensions and nasal and inhalation products. We’re also looking at niche API suppliers with complementary capabilities, especially in fermentation technologies, APIs and hormonal APIs
-
CAPEX is on track, spending in excess of 250 crores on the vaccine manufacturing facilities and these assets are almost fungible in nature (with little tweaks) can be used for biosimilar opportunites
-
Our fixed assets turnover also increased from 2.4x to 2.8x as we increase our capacity utilization
-
As of March 2021, we had total of INR 3000 crore of cash, which we intend to utilize for CapEx and to fund our organic and inorganic growth strategies.
-
As of March 31, 2021, we have 284 ANDA filings in US 1,501 product registered globally
Managment Changes
Ms. Naina Nal Kidwai (Harward Business school - rich experinece in banking & fiannce)
Dr. Alan Zhang - is a Scientist from the Parnet Company Fosun joined the board
@Rafi_Syed
In their presentation it’s mentioned at one place: Purchased assets of Vitane Biologics, Hyderabad based biopharmaceutical company.
Seems no information is available even on google search. Do post if anyone gets more info.
Q4FY2021 Conference call highlights
Results: The revenues for the quarter grew by 39.8% y-o-y backed by strong 29% y-o-y growth in the core-markets which include USA, Europe, Canada and Australia. The revenue growth could be attributable to new launches and volume growth in the existing product portfolio. Durring the period the company has new products including – Micafungin and Bivalirudin in RTU format as well as Olapatadine Ophthalmic product in the branded market.
Rest of World Markets: Revenues from the region grew by 196% y-o-y for the quarter attributable to new partnerships and increased geographic penetration. The company has forayed in new markets such as Singapore, Israel, Saudi Arabia, and CIS Countries
India Markets: The revenues from India business grew by 15% y-o-y backed by a ramped up supply of Remdevisir while reporting a healthy growth in Enoxaparin to cater to the requirements of the COVID patients
R&D: The R&D expenses for the quarter stood at Rs 30.4 cr as compared to Rs 17.3 cr in Q4FY2020. While for the FY2021 the R&D expenses stood at Rs 122 cr as compared to Rs 92.2 cr in FY2020.
Product pipeline and Launches: For FY2021 the company has filed 21 ANDA’s, 5 DMF’s and has received approval for 32 ANDA’s. As of FY2021 the company has a total of 284 filed ANDA and of this 234 are approved while 50 are pending approval.
Capex plans: For FY2021 the incurred capex stood at Rs 228.8 cr as compared to Rs 194.7 crore in
FY2020. Gland is expanding its sterile injectable facility located in Hyderabad and also will be investing
in the drug substance and biologics facility for creating robust infrastructure in vaccine and bio-similar
space. It is also enhancing its production capacity for APIs in Vizag and adding capacity in its oncology
facility so as to cater to the planned launches in forthcoming years. Accordingly Gland has earmarked a capex spend of Rs 300 crore in FY2022 and Rs 200 crore in FY2023.
Some developments on this stock:
- High entry barrier is reconfirmed as Sun Pharma in its concall says that they are not going to enter vaccine space
[No plans to get into vaccine production as it requires separate manufacturing infra: Sun Pharma - The Economic Times] - GST on Heparin & Remedesevir reduced to 5% , shall support Indian biz
- RasputinV Contract with RDIF is take or Pay as per Bernstein research report.
Hello,
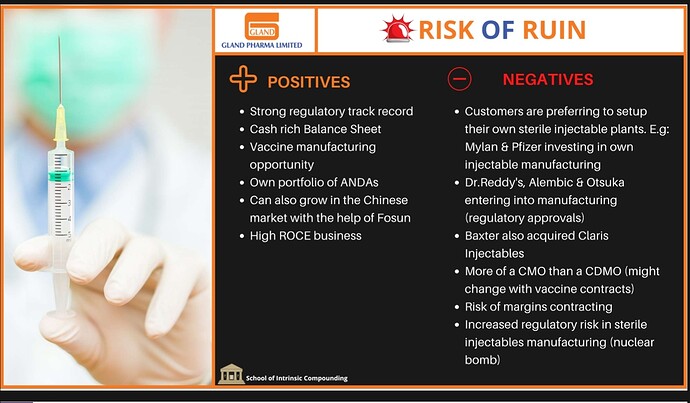
I was recently going through an API lecture by SOIC and saw a slide regarding Gland pharma with positive and negative points. It would be great if some of you can share your opinions on the same.
To start with, I don’t see other players coming to Injectable manufacturing soon. The reason is complex processes and high regulatory requirements. Sun pharma example provided above by Amitabh_Vatsya confirms the complexity issue.
The point regarding regulatory issues is actually confirmed by Baxter’s acquisition of Claris where they received a USFDA warning letter (link below).
At the same time, I agree with the point of being a CMO instead of CDMO, they are working on it with vaccine development but it’s still a long way to go.
If anyone can share more updates on these points, it would be a great help to us all.
Hi Sanjeev
Not an expert to comment but here are my thoughts
As you rightly mentioned (sterile ) it is a very high entry barrier (US FDA) business, it is not easy anyone just come in start the business. The regulations (sterile plant approvals) are very very stringent.
Aurobindo is unlocking its value
This is a long gestation period business even if someone wants to enter it takes long time to get approvals in regulated markets. Gland recently acquired one biopharma company to strengthen their vaccine manufacturing infra, they are also sitting on cash to do some inorganic expansion. They are about to start selling in China which in itself is huge market after US. Their last quarter growth in non regulated market is very very good, we have to keep few more quarters and see how this will aid the overall growth (Covid gave them an opportunity to enter some non regulated markets where they never had any presence ) , one more key thing is track their Chinese revenues.
Strong promoter backing (Fosun), excellent FDA record are the key strengths , Srinivas Sadhu (Ex Natco) like Rajeev N (Natco) is a man with clear vision.
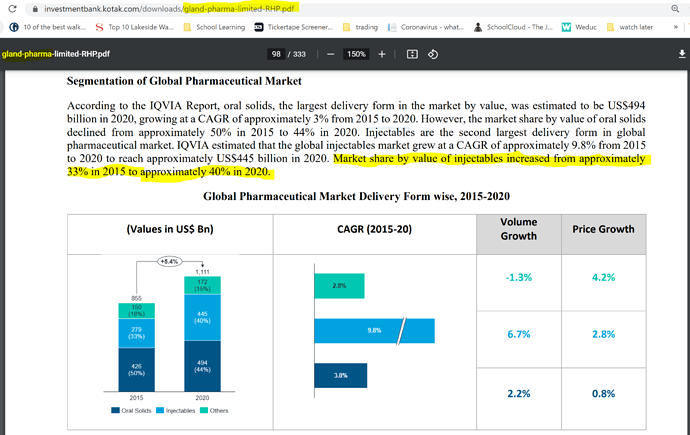
There is huge shortage of injectables in regulated markets, I will dig for a report and post here, so the market size is huge and it is not easy for anyone to enter and grab it, it is big pond opportunity is there for everyone.
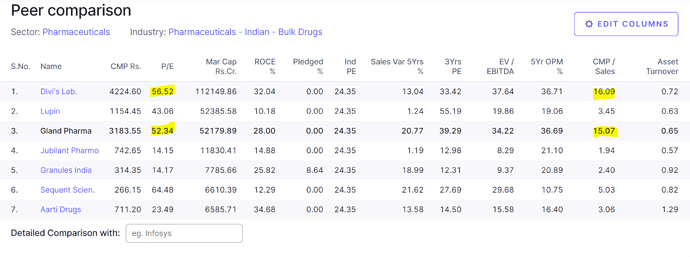
Valuations are super expensive , not a apple to apple comparison but with in the sector it is commanding the premium with the likes of Divis , there is no margin of safety left to take a fresh position.
Any idea why their receivables are so high? In the concall, Mr.Sadu also mentioned they will remain high. What is so different about their business model which is not there in other pharma companies?
I think he did reply to that query, this is due to more revenues from non regulated markets where the industry structure is like this (i.e receivables days are high )
Management has acquired 281,150 shares for only 1523.83 lakh rupees via ESOPs. It means each shares costs only 542 rupees to the management, is it normal for ESOPs to be at such a heavy discount to Market price? Does that signify that company is very Richly valued right now and price of 542 is closer to the real value of the company?
This ESOPS are given in March 20 and May 24 .2019 .just copied the details from RHP
You can have more details in RHP page 74 onwards
“Our Company, pursuant to the resolutions passed by the Board and the Shareholders of the Company on
March 20, 2019 and May 24, 2019, respectively, adopted the ESOP Plan 2019 and the ESOP Scheme
2019. The maximum number of shares that may be issued pursuant to the exercise of options granted to
participants under the ESOP Plan 2019 and the ESOP Scheme 2019 shall not exceed 17,044,400 shares.
Upon exercise and payment of the exercise price, the option holder will be entitled to be allotted one
Equity Share per employee stock option. The maximum number of Equity Shares that may be issued on
the exercise of all outstanding options granted under the ESOP Plan 2019, the ESOP Scheme 2019 and
any other share option plan or scheme of the Company, shall not exceed 30.00% of the number of relevant
class of shares from time to time. Further the ESOP Scheme 2019 provides that the maximum number
of options granted to any grantee shall not exceed 1.00% of the number of relevant class of shares in
issue (excluding outstanding warrants and conversions) at the date of the grant. The objectives of ESOP
Plan 2019 and the ESOP Scheme 2019 are, among others to reward employees for past as well as future
performance, link interest of employees with Shareholders, foster ownership and reward for loyalty. The
ESOP Plan 2019 and the ESOP Scheme 2019 have been framed in compliance with the SEBI SBEB
Regulations. As on the date of this Red Herring Prospectus, 1,549,500 options have been granted by our
Company under the ESOP Plan 2019 and the ESOP Scheme 2019. The details of the ESOP Plan 2019
and the ESOP Scheme 2019 are as follows:”
Read page 74 onwards in RHP I think price fixed before RHP filing
Thanks
Many companies are giving ESOPs even at Face Value… sad but true.