yes holding and tracking from 2.5 years
Me too- I took a position earlier this week.
Following it since its first concall. Added some more in OCT 2023. As management is delivering
I started investing around late 2023 as well. I’m trying to understand how the EBITDA guidance is 10% when the revenue guidance is set at 15%. Is there a strategy to shift toward a lower-margin product mix, even though the product mix plan seems different? I’m a bit unclear on this—does anyone have insights on it?
I believe the lower growth rate guidance for EBITDA was considering the near term pricing headwinds for Xanthine due to China
Q3FY25 Summary
Financial Performance
- Revenue: ₹538 Cr, up 20% YoY
- EBITDA: ₹129 Cr vs ₹96 Cr YoY, reflecting a 34% YoY growth
- EBITDA Margin: Expansion due to a higher contribution from CDMO/CMO businesses, solar energy project activation, and ₹7 Cr dividend income
- PAT: ₹74 Cr vs ₹53 Cr, up 40% YoY
Business Highlights
Xanthine Derivatives
- Recorded highest-ever quarterly volume sales, contributing 44% of total revenue
- Despite price corrections in spot markets (down by over $1/kg), the company’s long-term contracts and world-scale capacity helped mitigate competition from Chinese suppliers
- Facilities operated at full capacity during Q3
- Brownfield expansion progressing, expected to increase capacity from 5,000 MT to 9,000 MT per annum
API and Intermediates Business
- Achieved best-ever quarterly revenue performance, contributing 42% to turnover
- Revenue distribution: 48% Regulated Markets, 40% ROW, 12% Non-Regulated Markets
- The company continues to prioritize regulated markets for better profitability and long-term stability
- Tarapur API site driving growth, with Block 5 running at full capacity
- Adding a new production line to remove bottlenecks and increase capacity by 10%
CDMO/CMO Business
- Contributed 13% of turnover in Q3, reflecting the growing importance of this segment
- Currently working with 21 customers, adding 2 new customers this quarter
- Active projects: 56 projects (28 in commercial stages, 28 in development stages)
- Confident of delivering significant medium to long-term growth
Key Growth Initiatives
- Tarapur Unit 3 Regulatory Filings: EU GMP and CEP filings for pharmaceutical markets
- Greenfield Project at Atali, Gujarat:
- Phase 1 to be commissioned in Q3FY26
- Capacity of 450 KL across 58 reactors
- Designed for pilot to commercial scale-up
- Focus on Intermediates and CDMO/CMO production
- Renewable Energy Projects:
- 21 MW DC solar project commissioned at Akola, Maharashtra
- Signed 9 MW DC solar project for Gujarat unit, expected to be commissioned by Q2FY26
- Combined projects to cover 50% of total energy requirements
- Multi-fuel boilers capable of using bio briquettes to reduce fuel costs and carbon footprint
Business Dynamics and Outlook
- CDMO business expected to exceed ₹100 Cr revenue in FY25 (currently ₹77 Cr)
- API production at 75% capacity utilization, with new production line to increase capacity by 10%
- Focus on high-value niche product categories like anti-hypertensives, steroids, and anti-cancer APIs
- Regulated market share expected to improve as new products from the pipeline become commercial
Guidance
- Optimistic to exceed previous 10%-12% EBITDA growth guidance for FY25
- Confident of achieving 90% capacity utilisation of 9,000 MT Xanthine capacity by FY27
Challenges
- Temporary shutdown of Vapi plant in January due to pollution control board notice
- Estimated impact: <5% of turnover and ~2% of profit
- CDMO sales pushed by one quarter, but the long-term outlook remains intact
Management Insights
-
Xanthine Price Strategy: Offering different price proposals for different customer profiles, with spot market exposure capped at 30%-40% post-expansion
-
API Margins: Benefiting from 70% backward integration and niche product portfolio, minimizing China dependency
-
Regulated vs ROW Market: Higher margins from regulated markets, with ROW (China, Brazil, Russia) offering better prices than Europe and the US
Please suggest any site to track price movements of Xanthine derivatives
The real play is not Xanthine derivatives anymore; its the CDMO business It’s better to focus on how that will play out.
Xanthene will always a remain a large part of the biz. The CDMO biz is just RSM, KSM - doesn’t matter the number of molecules it will never be able to reach the scale of operations of pure play API cos. Look at the gross margin profile, also pharma grade Xanthene is also a great optionality in the biz
Can anyone suggest how to track price movement on Xanthine derivatives manufactured by AARTI
From here
- ICIS - https://www.icis.com
- ChemAnalyst - https://www.chemanalyst.com
- TradeIndia - https://www.tradeindia.com
What is RSM - KSM
Disc: Newbee investor. Holding tracking position
A lot of ingredients go into making a drug
KSM (key starting material), AI (advanced intermediates), API (the most imp part of the drug - the one which brings the therapeutic effect)
KSM and intermediates are into the backend of the value chain in the manf of the drug and thus cos that manf them for other cos (even in case of innovators - the ones who create the new drugs) have a lower gross margin - just check the gross margin of CDMO division of AMI organics and aarti pharma - probably around 45-50% range.
Pure play API cos like say Neuland for eg. have much higher gross margins - sometimes north of 70% - talking about material gross margins.
All you have to understand is no matter how many molecules you have in the RSM KSM (the starting materials to manf the drug) - the gross margins and the scale will always be much lower compared to pure play API companies where there’s much higher requirement in absolute volume and also command better gross margins as they are the most crucial part of the drug.
Rest some googling will help ![]()
Is there any reason behind promoter selling ?
Adding to Bharani’s masterclass on CDMOs:
I had a certain understanding of CDMO businesses:
- Treadmill business if you are very early in the CDMO journey. You have to keep finding new molecules frequently as supply to innovator is lumpy and your pipeline is small.
- Thumb rule – API will get 10% of the innovator sales. Intermediate will probably get 5% of the 10%. If you are doing end to end including formulation, you might get 12-15% including milestone payment. So, the innovator might make 85% gross margin.
- Valuation multiple for Innovator >>> CDMO
While Bharani has explained most of it using Neuland’s example, I felt Aarti Pharma is a live case study in the making.
Aarti Pharma has exported 2 intermediates for Bayer’s new drug Elinzanetant. Elinzanetant, formerly known as NT-814, is a dual neurokinin-1 and neurokinin-3 receptor antagonist under development for treating menopausal symptoms, particularly vasomotor symptoms such as hot flashes.
Note – Aarti Pharma mgmt. has not mentioned about this anywhere. It is my understanding that the 2 intermediates they exported in Q4 FY25 could be for Bayer’s drug. I could be wrong.
Bayer is a global leader in women’s healthcare. It acquired British biotech Kandy Therapeutics Ltd which developed NT-814. KaNDy was spun out of NeRRe Therapeutics, a GlaxoSmithKline spinout, in 2017. NeRRe filed the original patent for NT-814 / Elinzanetant:
Under the terms of the agreement, Bayer will pay an upfront consideration of USD 425 million, potential milestone payments of up to USD 450 million (for Phase-3 and regulatory expenses) until launch followed by potential additional triple digit million sales milestone payments.
The Phase III clinical trial is over and US FDA action date is July 2025. Once approved, the compound could generate peak sales potential of more than $1 billion globally.
https://www.bayer.com/en/ca/news/canada-bayer-to-acquire-uk-based-biotech-kandy-therapeutics-ltd
Note (for Wockhardt investors) – 425 Mn for a Phase-2 drug with a peak sales potential of >$1 Bn!
(Elinzanetant’s competitor drug Veozah was acquired for $588 Mn - https://www.fiercebiotech.com/biotech/bayer-inks-425m-upfront-kandy-buyout-to-challenge-astellas-for-menopause-market)
To understand the molecule, you have to read the patent fully. Or upload it to ChatGPT and ask the right prompts.
To put it in simple terms, there are 2 key intermediates to make Elinzanetant. Lets call them N-1 and N-2. The N-1 and N-2 intermediates are coupled together to form the API.
To calculate how much quantity of N-1 and N-2 are needed for 1 KG of API, we have to understand the Reaction Stoichiometry. The Elinzanetant synthesis follows a 1:1 coupling ratio. Each mole of N-1 reacts with 1 mole of N-2 to form 1 mole of Elinzanetant.
Given the molecular weights:
- N-1: 532.89 g/mol
- N-2: 262.35 g/mol
- Elinzanetant: 668.65 g/mol
In an ideal scenario with 100% yield, the mass balance would be straightforward:
- Combined Mass of N-1 + N-2: 795.24 g
- Mass of Product (Elinzanetant API): 668.65 g
This indicates that, theoretically, 668.65 g of Elinzanetant can be produced from 795.24 g of combined intermediates. The difference accounts for the loss of by-products such as water or other small molecules during the reaction.
However, actual industrial processes rarely achieve 100% yield due to side reactions, incomplete conversions, and losses during purification. Assuming an 85% overall yield, the expected recovery would be:
- Actual Yield**:** 668.65 g × 0.85 = 568.35 g of Elinzanetant.
Based on export data, Aarti Pharma has sent:
- N-1: 2,371 kg
- N-2: 2,142 kg
First, determine the limiting reagent by comparing the molar amounts:
- Moles of N-1**:** 2,371,000 g / 532.89 g/mol ≈ 4,451.6 mol
- Moles of N-2**:** 2,142,000 g / 262.35 g/mol ≈ 8,165.2 mol
Since N-1 has fewer moles, it is the limiting reagent. The theoretical amount of Elinzanetant produced is based on the moles of N-1:
- Mass of Elinzanetant API**:** 4,451.6 mol × 668.65 g/mol ≈ 2,976,000 g or 2,976 kg
Considering an 85% yield:
- Actual Mass of Elinzanetant API**:** 2,976 kg × 0.85 ≈ 2,529.6 kg
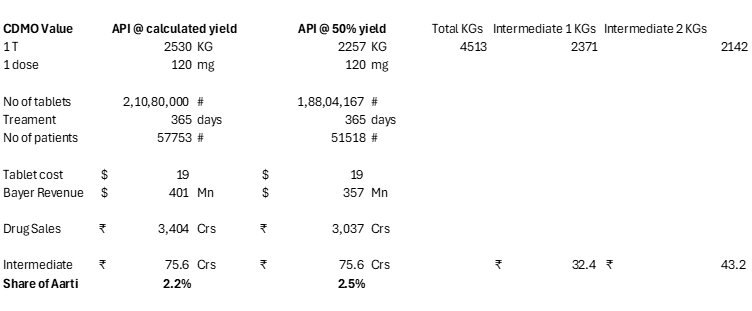
Therefore, approximately 2,529.6 kg of Elinzanetant API can be produced from 2,371 kg of N-1 and 2,142 kg of N-2. Yield = 2530/4513 = 56%.
Note – These estimates are based on online references and can be completely wrong as real world is very different and we are working with lot of assumptions.
So how many tablets can be made from 2530 KGs of API?
One dose is 120 mg based on clinical trials done by Bayer.
2350 KGs / 120 mg = 21 Mn tablets.
Elinzanetant’s competitor drug Veozah sells at roughly ~19$ per tablet. It is the list price that a patient without prescription insurance pays:
So Bayer’s sales from this drug will be = 21 Mn * 19$ = $400 Mn or 3400 Crs.
Aarti’s 2 intermediates were sold for 75 Crs.
So Aarti gets only 2.2% of the end drug sales.
As Bharani indicated, the dosage is one of the important drivers for the CDMO company’s value from the drug.
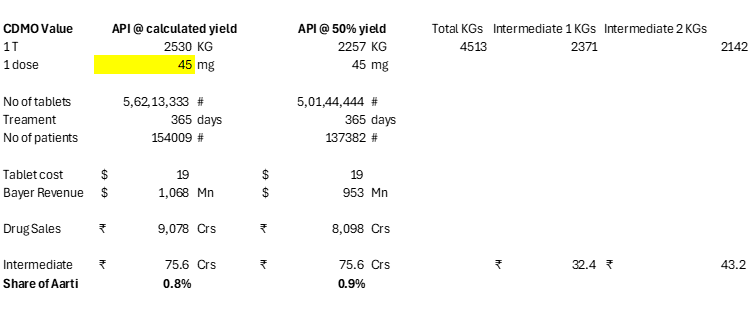
If I reduce the dosage from 120mg to 45mg which is Veozah’s prescribed dose, Aarti’s value falls to <1% as now Bayer will have to sell more tablets and hence more patients:
One positive for Aarti could be the yield. If the recovery of API during the final stage is lower than our assumption, Aarti’s value capture can be higher.
How many tablets does a patient take every day and for how long?
While the minimum period for Veozah is 3 months, the average can be upto 1 year. So a patient will need 365 tablets. 21 Mn tablets will be consumed by 58k patients.
Which means, Aarti might not supply more intermediates till Bayer sells to 58k patients. If you consider the consumption period to be lower than 365 days, say 250 days then Bayer has to sell to 84k patients. Because of this issue, it is better to track and benchmark absolute sales.
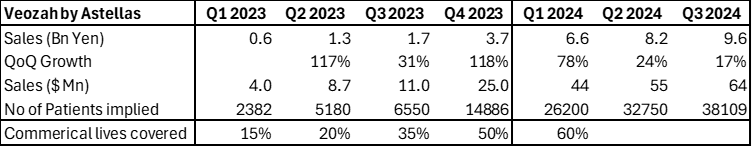
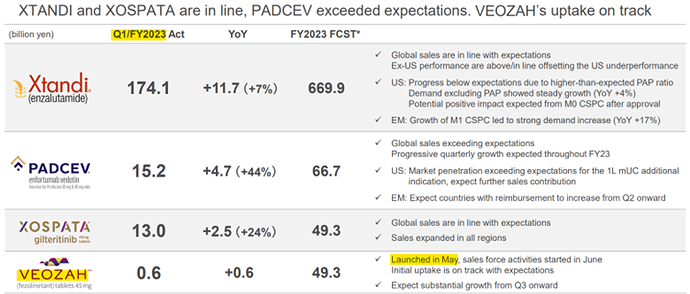
How has Astellas scaled with Veozah so far? Veozah was approved by US FDA in May 2023 and EU in Dec 2023.
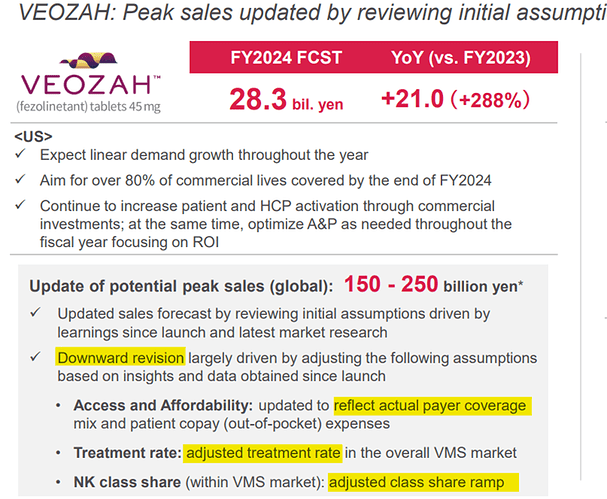
Veozah has reached a quarterly sale of $64 Mn in the 6th full quarter since launch! It was not a clear path to it.
When they launched, they had forecasted 49 Bn JPY or 325 Mn USD for FY23 but actual in FY23 was only 7.3 Bn JPY or 50 Mn USD!
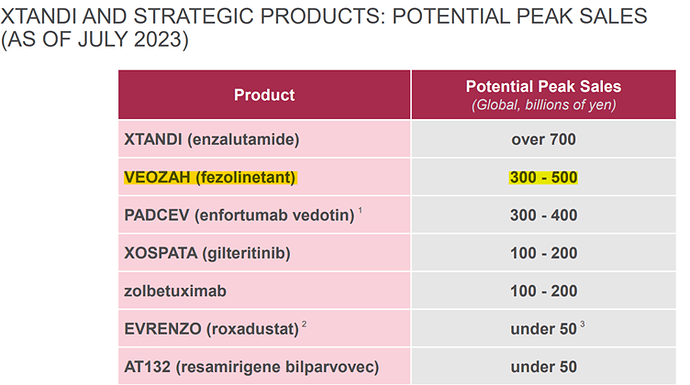
Initial projection of peak sales potential of 300-500 Bn JPY or 2-3.33 Bn USD! ![]()
But within few quarters, they had to revise it downwards to 150 – 250 Bn JPY or 1-1.66 Bn USD.
Reasons for downward revision:
- Insurance – payer coverage / out of pocket expenses – During launch, Veozah was eligible for only 15% commercial lives.
- Treatment rate
- Class share – within the treatment options, rank of drug.
Late last year, Veozah hit a small roadblock. In Sep 2024, US FDA issued a warning that Veozah can cause rare but serious liver injury. In Dec 2024, FDA added a Box Warning:
Coming back to calculating time taken by Bayer to utilize the intermediates sent by Aarti:
Astellas did 220 Mn in 6 quarters. Bayer being a global leader in women healthcare can do it in 3-4 quarters? But the API potential based on our assumptions is 400 Mn! If we assume Bayer to consume 400 Mn by 6th quarter from launch (Q2 FY26, our FY), then they don’t need more API till Q4 FY FY27. If Bayer buys intermediates 6 months before next batch of API needed, then Aarti will supply its 2nd batch only in Q1 or Q2 of FY27.
Best case for Aarti Pharma in my opinion assuming 1) insurance coverage increases significantly, 2) additional indication for breast cancer hot flashes, and 3) Veozah loses market share, Bayer will not need intermediates till Q4 FY26 at least.
Very good article on all issues regarding Veozah ramp up:
- Payer reluctance / stringent requirements (like it has to be prescribed by ob/gyn and patient has to try other non-hormonal treatments first) - https://healthy.kaiserpermanente.org/content/dam/kporg/final/documents/formularies/nw/kp-veozah-nw-en.pdf
- General awareness (menopause symptoms are not considered a disease)
- Patient and doctor acceptance (which is mostly side effects and insurance cover)
Hopefully, awareness and acceptance only increase from here on. And Bayer will have a second mover advantage from all the spending that Astellas has done so far (Example - https://www.youtube.com/watch?v=e-H2BF0GZik) in market creation. Bayer might also have a higher insurance coverage for its drug compared to Veozah during launch.
Summary:
- Treadmill business. Yes, Aarti Pharma even in the best case will not supply intermediates to Bayer till Q4 FY26 at least. Has this happened before? Yes, Yes, Aarti had supplied 4500 KGs of an intermediate for the drug Osimertinib in FY24. In FY25 so far, they have supplied only 2250 KGs. Similarly, intermediate for Afoxolaner supplied in FY24 was 23500 KGs but only 9900 KGs in FY25.
- Value for CDMO. Aarti Pharma might get 2-2.5% of the drug sales. So even if Bayer hits $1 Bn in Elinza, Aarti Pharma from the current 75 Crs will move to ~200 Crs supply. By when can it happen? Best case - Maybe 2-3 years from launch, so FY28 with the risk that in FY26 there can be no further supplies? Despite the lumpiness, potential of ~2.5x in 3-4 years is very good. Have to keep tracking the data.
- Initially, I was of the view that innovator will have only 85% gross margins. With the current understanding, it is possible that the innovator can have 95% gross margins too especially in acute therapies. 2% Intermediates, 2% API (can be higher too?!), 1% formulation = 5% COGS. So, the incremental 10% has to go to the innovator? What does it mean for an Indian innovator who is looking to out license the drug? In this Wockhardt post - Wockhardt: an NiCE story - #25 by Sanjay_Kumar_E – I had assumed only a 15% royalty for Wockhardt and 20% PAT margin for the buyer. Both can be significantly higher. Wockhardt can get 20% royalty and if they are lucky, 25% too. And Wockhardt’s royalty will be consistent because it is not linked to drug manufacturing but the actual sales.
Going through the patent and the manufacturing process also gives some indication of the capabilities that these CDMO companies have to make the intermediates or APIs.
In this case, ChatGPT says N-1 involves Fluorination (I don’t know if Aarti imports RM post fluorination or if it does it) and N-2 involves Cyclization & Hydrogenation. API process involves Nucleophilic substitution, Hydrochloride salt formation and Deprotection. I am not sure if the API has more value addition (ChatGPT says yes) than the 2 intermediates (was trying to see if this molecule is also similar to Bempedoic Acid where Bluejet which makes intermediates adds more value than Neuland which makes API) but more importantly @aga.ayush11 says that CDMO companies win certain molecules based on their chemistry and process capabilities.
Disclaimer:
I am a rookie analyst. I have been studying the CDMO space in some depth only over the last few months across companies. I am not professionally qualified in this field and by no means an expert - so I am bound to have made lot of mistakes. ChatGPT could be wrong too. This post is a case study to understand CDMO value chain with a live example.
Aarti Pharmalabs wins - Good Documentation Practices Award (Within Rs 1000 – 2000 Cr.): Aarti Pharmalabs (Unit IV Tarapur)
Note: Invested (holding tracking position)
Q4 FY25 Concall Updates
- Long term relationships and repeat orders from three main customers are very important for their steady business.
- Expect EBITDA to grow by about 12% to 15% in FY26 over the high base of FY25 due to increase in contribution from higher margin products and improved process efficiencies and volume growth. The lower guidance is because of the expenses that will start adding up once Atali is commercialized.
- Xanthine production facility (9000 MT) is expected to be fully up and running by early next year. In the next three years, they aim to use 80% to 90% of this new capacity, with about half of the sales going to beverage companies and regulated pharmaceutical customers.
- Expect the revenue from CDMO/CMO business to grow by 30% to 40% in FY26. Current mix of approximately 60 products and 21 customer, their recent success in adding about 20 new products and six new customers in the last year
- Roughly around 70-75% of the 33 commercial projects (CDMO/CMO) contributed to revenue in FY25
- Mechanical completion of Atali Phase I is expected by end of Q1 FY26 while it’ll take 3-4 quarters more for regulatory clearances and commercial supply readiness
- The corticosteroid product range is very stable. While there might be minor price adjustments, since these products are typically sold in limited quantities, customer stickiness is there and hence it’s a continuous and stable business.
- For the API segment overall growth is driven by regulated markets and export-backed markets
- US exports are not a very significant portion of their total revenue and APIs are exempt from import duties and even if there is any duty impact it will be on formulation and not API.
- Total capex for FY26 to be around 400-450 crores. This includes the remaining spending on the Atali project (200 crores) and the Xanthine expansion (100 crores plus) and rest in maintenance and R&D capex.
- Xanthine derivative prices are currently stable, although low. The management believes that prices have bottomed out and there would be slight increase in overall Xanthine volumes in FY26 due to phased commissioning.
- The bulk of CDMO sales (75%-80% of total sales value) comes from products in Phase 3 onwards and they expect certain products from Phase 3 to be launched in the next couple of years.
- Also, actively attempting to work with higher molecular weight products, including mid-size peptides
- The new Atali plant will also include intermediate manufacturing. Additionally, at Tarapur unit 4 API site, they are improving an existing block by adding an extra production line which will increase their overall capacity for general products by about 15-20%.
- The business Aarti USA conducted for Aarti Industries is expected to decrease however it’s not winding down.
- There is a 20% difference in realization between pharmaceutical and consumer-focused Xanthine with 54% of their total volumes last year was for beverages while the rest was for pharmaceutical use
- Ganesh Polychem business experienced a slowdown in overall demand and they had produced a large quantity in an earlier quarter. Also, they are upgrading the facility to enable a lower-cost production method for the products, which has led to a shutdown lasting almost three to four months. Because of this shutdown, the numbers for Q4 and Q1 of the next financial year will be impacted however after that it could hav margin expansion.
- Expect the borrowings to increase by about 100-125 crore in the current year
Disclaimer: Studying