Here is my latest summary of company Aarti Pharma Labs Limited - Harnessing Innovation and Expansion for Sustained Growth
Executive Summary
- Expansion of new facility to boost revenue.
- Additional clients in CDMO & CMO segment to add to margins.
- Professional management team running day to day operation along with promoters removes key person risk.
About
The company is engaged in the manufacturing of APIs, intermediates, and xanthine derivatives. With a strong focus on quality and innovation, the company serves global markets, including North America, Europe, and Asia, offering a diverse range of healthcare products.
The company has over 500 global clients, including Liconsa Laboratories and Caribbean Refrescos, as well as domestic customers such as Dr. Reddy’s Laboratories, Zydus Healthcare Ltd, and Glenmark Pharmaceuticals. It was demerged from Aarti Industries in October 2022 and got listed on the stock exchanges on January 30, 2023.

Product Portfolio -
-
Xanthine Derivatives (52% of revenue)
Industry scenario
Xanthine Derivatives include Caffeine, Theophylline Anhydrous, Aminophylline, and Etophylline which are used for asthma-related medicines. It also finds applications in
- Beverages (70-80% of consumption) -
- Nutraceuticals, and pharmaceutical industries (20-30% of consumption)
Company
The company commands 15-20% global market share & 80% domestic market share in Xanthine. It has the lowest margins compared to CDMO & API & intermediates.
The global competition in the segment comes from Chinese players which can quote lower prices because of the scale at which they operate. The prices of Xanthine derivatives have reduced by 15-20% along with a fall in raw materials in recent times. The management of the company believes the prices have bottomed out & won’t reduce from hereon.

-
API and Intermediates (36% of revenue)
The company mainly does low-volume and high-value drugs. This includes antihypertensives, anti-diabetic, steroids, and oncology drugs. The revenue contribution from the regulated market is 75-80%.
The company’s facilities are accredited by the USFDA, EU GMP, EDQM (European Pharmacopoeia), KFDA (Korea), and COFEPRIS (Mexico). It also has 500+ global clients like Liconsa Laboratories, and Caribbean Refrescos and domestic customers like Dr. Reddy’s Laboratories, Zydus Healthcare Ltd, and Glenmark Pharmaceuticals.

-
CDMO & CMO (11% of revenue)
The revenue is majorly driven by the Contract Manufacturing Organization (CMO) compared to the Contract Development and Manufacturing Organization, where 80-90% of the business is driven by manufacturing intermediates.

This segment has the highest margins compared to the other 2 segments as the company starts working from the pre-clinical and early phases. For intermediates and KSM RSM, they start working from late phases with innovators. They work directly and indirectly (through API manufacturers) & the innovators make the final decision on whom to source from. Once initiated, CDMO projects take 3-4 years to provide peak revenue. The company is currently engaged with 19 companies on 53 projects, whereby 27 are commercial and the rest are under development at the customer’s end (13 added in Q1FY25)

Manufacturing footprint

- Xanthine
Existing Capacity -
The current capacity is 5 KTPA (increased from 4 KTPA) which is spread across 2 units at Dombivali & is currently operating at a 90% utilization rate.
Capacity expansion
The company is acquiring land near the existing facility for expansion purposes. The total Xanthine capacity will increase from 5 KTPA to 9 KTPA over the next 2 years across 2 sites. The cost of the expansion is estimated to be around Rs. 150 cr.
The new upcoming facility is already tied up with the customer as clients want to increase their sourcing from the company. The management expects the new expansion to be fully utilized by the end of FY27.
- API & Intermediates & CDMO
Existing Capacity
These capacities are interchangeable with CDMO capacities & currently operating at an 85% utilization rate. Vapi and Dombivli sites primarily manufacture intermediates for CDMO & Tarapur site manufactures API’s for CDMO.
Capacity expansion
The company has planned capex of 425 cr in total which would be primarily utilized for
- Land - 50 cr for 80 acres of land. There would be enough space for 1000cr of further capex which would be planned over next 5-7 years.
- Infrastructure - 175 cr will be spent for building infrastructure & 2 blocks
- Plant & machinery - 200 cr for plant & machinery for 400 KL of capacity
The new capex will have a peak revenue potential of Rs 375 - 450 cr
Solar power plant in Akola
The company established a 20 MW solar plant for Rs 80 cr ( estimated based on 4 cr per megawatt cost of the solar plant). This help bring power costs down by ⅓ for the company.
The company expects a 4-5 years payback period and ROI of 20% from the project with the project commencing operations in September 2024.
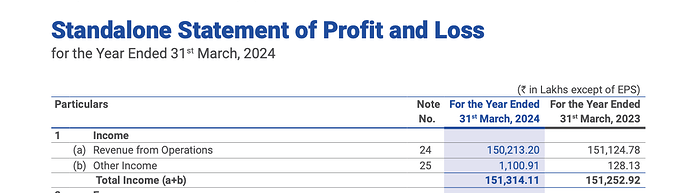
Financials & outlook
|
2023 Actual |
2024 Actual |
2025 Expected |
2026 Expected |
2027 Expected |
| Revenue |
1945 |
1853 |
2131 |
2451 |
2818 |
| Revenue Growth Y-o-Y |
|
-4.73% |
15% |
15% |
15% |
|
|
|
|
|
|
| EBITDA |
342 |
386 |
425 |
467 |
514 |
| EBITDA Growth Y-o-Y |
|
12.87% |
10% |
10% |
10% |
|
|
|
|
|
|
| EBITDA Margin |
|
20.83% |
19.93% |
19.06% |
18.23% |
|
|
|
|
|
|
| Current Enterprise Value |
5573 cr |
|
EV/ EBITDA |
13.5 |
|
| Current Market cap |
5228 cr |
|
P/E |
22.9 |
|
The growth guidance of revenue & EBITDA has been taken based on management guidance of 15% revenue growth & 10% EBITDA growth on a yearly basis.
The company expects CDMO share to increase to 20-25% of revenue in the next 2-3 years which would further boost the EBITDA margins of the company which haven’t been accounted for currently. The company gave EBITDA guidance of 15% in FY24 which was duly achieved by the company.
Management
-
Rashesh Gogri, Chairman - He is a dynamic leader and second-generation entrepreneur, who has lead leadership at Aarti Industries Ltd. A graduate of production engineering from Mumbai University, his profound expertise and strategic foresight have been pivotal in the execution of numerous transformative projects. He has played a key role in the growth of various strategic business units in the chemical, pharma, and personal care segments.
-
Hetal Gogri, Vice Chairperson & MD - A second-generation entrepreneur, she joined the Aarti group in 1996. Joining the company’s board in 2001, she has held leadership roles in procurement, marketing, and supply chain functions, and has managed various strategic business units. Leveraging a rich experience of over two decades in the chemical and pharmaceutical industries, she has been a key contributor to the company’s growth. Following the demerger of the pharmaceutical business from the company, she has been serving as a Managing Director since 2022.
Competition
Xanthine
The biggest Xanthine producers worldwide are from China who command ~ 70% global market share. Germany has a sizeable player (10% global market share) with capacity similar to Aarti Pharmalab limited (20% global market share). Also Chinese players are expanding their Xanthine capacity.
Any new player requires 2-3 year of gestation period in order to get validation & approvals from the customer.
Also, Xanthine faces competition from Synthteic caffeine which is manufactured by -
- CSPC Pharma Group
- BASG SG
- Spectrum Labs
- Kudus Chimie
- Shi Yao Pharma
- Minerals Limited
- Shri Ahimsa Mines
- Micro Labs India
Risks
- Closure of facility - Previously, the company had some issues with the boiler which led to a pollution control board notice & closure of the plant. The plant was restarted within a month but in future, if company isn’t able to maintain pollution norms the company faces risk from the government for plant closure
- Dumping by Chinese players - If the situation of overcapacity is created in the market, then there is a possibility of dumping from Chinese players which might reduce the realization & the margins of the company.
Conclusion
The company’s product portfolio, which includes high-demand Xanthine derivatives where the company holds the pole position in India, APIs, and intermediates, positions the company for sustained future growth. The key monitorable remains the supply side of Xanthine in the market & new wins in CDMO & CMO which the company expects to become a major growth driver, & boost margins as it commands the highest margins within company’s product mix.
The shift due to the China+1 strategy & Biosecure Act might prove beneficial to the company as the products are well-placed alongside the necessary approvals which makes the company tick the right box for future growth. The boost in the growth would be due to the company’s strategic expansion plans, such as increasing Xanthine & API & intermediates production capacity along investing in solar energy, which will further enhance profitability.