(post withdrawn by author, will be automatically deleted in 24 hours unless flagged)
(post withdrawn by author, will be automatically deleted in 24 hours unless flagged)
Please discuss portfolio related stuff in the portfolio thread. Do not clutter Ajanta thread.
Ajanta receives USFDA approval for Irbesartan (75, 150 and 300mg). Used for treating hypertension and nephropathy in type II diabetic patients.
http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=203685&TABLE1=OB_Rx
The total market size for Irbesartan tablets as per IMS is ~$50 million per annum.
Sanofi, Ajanta, Alembic, Apotex, Auro, Cipla, DRL, Hetero Jubilant, Lupin, MacLeods, Mylan, Prinston, Roxane, Sandoz, Sciegen, Teva, Unichem, Watson, Zydus have Irbesartan approval.
Getting FDA approval alone is not enough but making it profitable is the real test. I think Ajanta Pharma is one right track in getting approval for the drugs which has the potential of profitability rather than just for the sake of approvals.
Deleted. posted at Torrent
Deleted…Posted at Torrent
Deleted…Posted at Torrent
good correction in Ajanta, anybody adding up
I bought today around Rs 1255 to the tune of 5% of my PF worth. I’m averaging up from Rs 360 levels. Will keep buying more if there is any “Ajanta Discount Sale.” 
Trading at less than 30 times forward PE and looks fair for a 15-20% growth predictable business with US business kicker yet to play out.
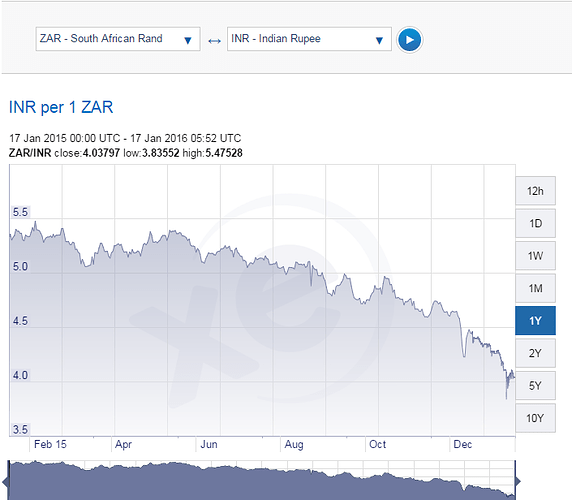
Does any one correlate the reason for recent fall from 1450 to 1170. All most 25-30% in 2 months.
I assume that ZAR vs INR fall from 4.7 to 4 might be the reason as most of the revenue around 60% from south Africa and recent correction on USD to INR might highly impact on revenue etc.
Is it correct correlation or I am missing something ?
I had asked co’s IR last yr about the impact of African currencies when Nigeria currency depreciated by around 30%. He replied that they book revenue in USD and depreciation of african currencies have no impact on Ajanta.
As per my knowledge, all global wire transaction happens on USD currency. So first domestic currency converted against USD and USD transfer to India INR. So is it correct or they are doing some hedging the currency like IT company or ?
Let me know if you have any further info about currency impact.
My sense is that if they book revenues in USD, they essentially quote and receive funds in USD. In that case the only impact a weakening ZAR can have is lower purchasing power of South Africa.
The drop in Ajanta’s stock price does not surprise me much as 45-46 PE was outrageous.
I am more comfortable buying again at 25-30 PE.
The major driver of PAT growth over recent years was margin expansion which seems to have topped out. I am not saying that there may not be a 4-5% variation going forward, but margins look stabilized now compared to other pharma cos.
My thesis of investing in Ajanta is the revenue growth which looks to be compounding at 25%-30% CAGR. This alone is sufficient to make it a good investment bet at 25-30 PE.
Further margin expansion, if it were to happen, will be an icing on the cake.
Hi Sushil,
I think a PE of 30 is quite attractive for Ajanta not only because of the 15-20% growth in profits, but also on account of the exceptionally high RoE of the company.
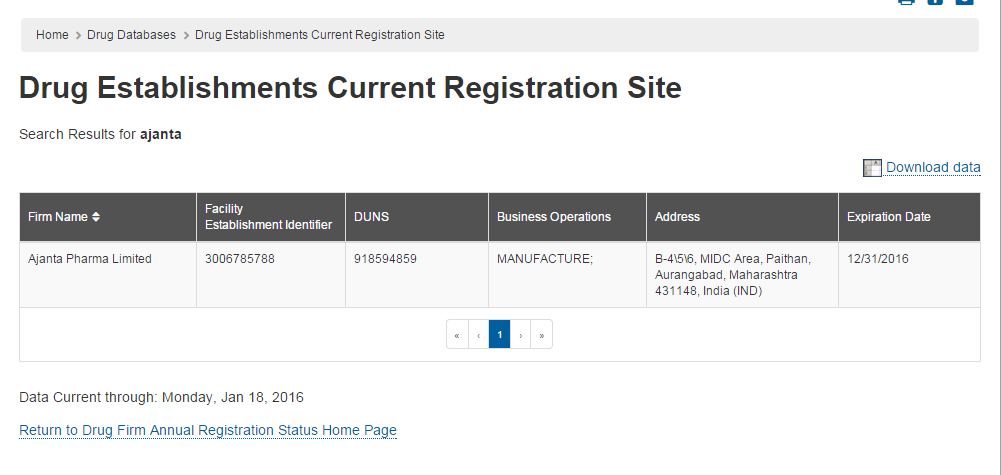
Any idea why Ajanta’s Dahej plant is not showing up here:
http://www.accessdata.fda.gov/scripts/cder/drls/default.cfm
I tried torrent and their recently commissioned dahej plant is listed.
From USFDA site - Drug Establishments Current Registration Site | FDA
Establishments must be registered within 5 days of beginning operations. (21 CFR 207.21(a) and 207.40 and FD&C Act sections 510(c), (d), & (i)). In addition, establishments must renew registration annually between October 1st and December 31st of each year. (FD&C Act sections 510(b) & (i)).
Firms that send their initial or annual registrations during October 1st to December 31st period are considered registered until the end of following year. If a firm submits its initial, updated or annual registration outside this time frame, it is considered registered until the end of the current year and shall renew before December 31.
Most likely scenario is -
They may have registered between Oct-Dec 2015, due to which they need to renew the registration for 2016.
What we do know is the following:
- Unlike Torrent which had FDA Inspection on April 2015, Ajanta has not had that 1st inspection yet.
- But since they are sending regulatory filing batches, they would have been allotted a FEI (Facility Establishment Identifier) which would imply they may have been registered.
- As for Torrent, since they had started commercial operations in Nov 2015, they would have been expected to register within 5 days as stated above.
I may be wrong with my assessment and others can correct me.
Sounds like a possibility. Thanks!
Hi,
I was going through the BS of ajanta pharma and observed that their fixed assets haven’t increased much in last three years . Does that mean their assets are getting old and will need to invest more in coming years
-
2014. 2015
262.73 261.4 271.32
Source: screener.in
Ajanta gets ANDA approval for Sildenafil Citrate
http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails