Torrent has only for EQ 20 mg base, but Ajanta got tentative approval for multiple strengths. does it mean Torrent has to apply again for other strengths?
Its tentative approval and not final approval.
Q3 FY16 Highlights
- Revenue from Operations grew 16% at 473cr against Rs 408cr (YoY).
- Profit before tax grew 13% at 152cr against 135cr (YoY).
- PAT grew 20% at 111cr against 93cr (YoY). (Note: Relatively less Tax paid this qtr)
- The currency devaluation and scarcity of forex availability in few emerging markets limited us to achieve our full potential.
- Making substantial investments in R&D and manufacturing facilities to build required infrastructure to fuel growth plans.
India
- India branded business grew 17% YoY to 128cr.
- Total India revenue grew 1% YoY to 134cr.
Emerging Markets
- Business grew 22% YoY to 328cr.
- Pipeline of 1,800+ products under registration paving the way for sustained growth.
Regulated Markets
- 7 final and 2 tentative approved ANDAs.
- 4 products have already been launched.
- Another 17 ANDA’s are in various stages of approval with USFDA.
R&D
- R&D spend has increased significantly during the quarter.
- About 5% of net sales.
- ln 9m FY16, the total R&D expense (excluding capex) was 70cr against 41cr for the same period last year.
Manufacturing
- Solid dosage formulation facitity at Dahej has started taking regulatory filing batches.
- Commenced the construction of new formulation facitjty at Guwahatt with proposed investment of 300 cr and plan to operationalize it before Mar 17.
Thank you @lustkills for posting result details. Good thing is they are getting good traction in regulated markets.
Co has updated Investor presentation.
Here is the link
http://www.ajantapharma.com/financials/Investor_Presentation/Q3-15-16.pdf
Any reason for slowing growth in Domestic markets?
Two reasons -
- Slowdown in key Derma brand Melacare. Management has trimmed down domestic growth expectation in Q2FY16.
- Moving away from low margin Domestic Institutional business (down 75% YoY to 7cr) to continue focus on Domestic Branded formulations (up 17% YoY to 128cr).
Ajanta’s USP has been knack of launching maximum number of first time launches. Of 188 actively marketed brands, 129 brands were first in India. Just need to continue this trend to get back to ~25% domestic growth.
Ajanta pharma I-sec recommendation - Target Rs 1780
Ajanta pharma ISec recommendation 03 02 2016.pdf (335.8 KB)
“… Our target is to file more than 10 ANDAs every year and we are looking to file the products where there is a limited competition kind of scenario which are more complex and extended release, delayed release, controlled release, those kind of formulations. We are very poised to do those filings next year, particularly with the Generic Drug User Fee Amendments (GDUFA), the commitment from the FDA, to approve the ANDAs as per the predefined timeline. So, the approvals should come fast and we should be able to make those ANDA approvals, convert them into the numbers faster than before…”
- Yogesh Agrawal (on the sidelines of the Antique Investor Conference)
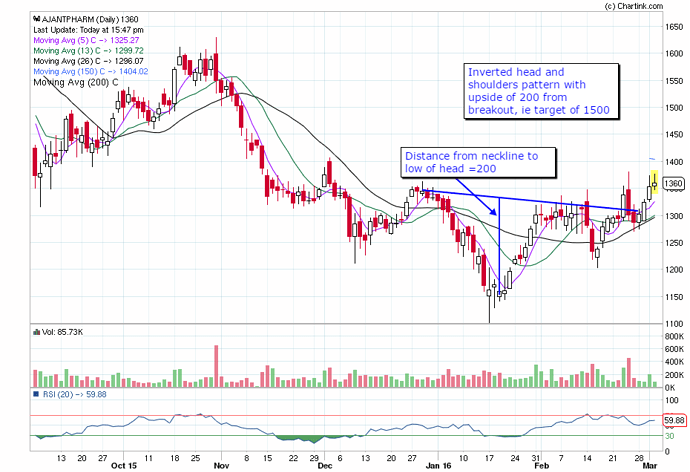
@hitesh2710 Please let me know if my reading of inverted head and shoulder pattern on ajanta is correct.
pikrohit,
It is better visualised on weekly line chart.height of pattern is around rs 150. A similar pattern also is present in alembic weekly line chart though the height of the head is much smaller.
It is raining dividends.
Ajanta Pharma Ltd has informed BSE that a meeting of the Board of Directors of the Company will be held on March 09, 2016, inter alia, to consider and approve declaration of interim dividend for financial year 2015-16.
http://www.bseindia.com/corporates/anndet_new.aspx?newsid=75b04c55-d500-43dc-86ee-d152d18e6a59
Breakout from HnS requires high volume compared to the volume during HNS formation. In this case, volume has not risen on breakout.
Hi,
Dont consider any stock chart in isolation. Always, relate it to the Nifty levels. When Nifty is falling, no stock pattern can succeed. For your information, Traders and operators use FIBO levels rather than the charts.
There is a W pattern with Nifty @ 7500. If 7500 resistance holds, all stock patterns will fail.
Always align your long or short in any stock with Nifty as it is a mother of all charts.
@hitesh2710 thanks for comments
@Ishank…I thought that the volume at breakout candle should be higher than the previous volume. I checked that this holds true for daily charts but not weekly chart
Ajanta get approval for gAxert (almotriptan malate). Link - http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails
Almotriptan is prescribed to treat the acute headache phase of migraine attacks. This is the first approval for a molecule for Ajanta where the market is not crowded with just 2 other generic players in the market currently including Teva and Mylan. However, the market size of the drug is small at around USD 31 million (source - http://www.businesswire.com/news/home/20150708006211/en/Teva-Receive-Approval-Launch-Generic-Axert®-Tablets).
Ajanta declared 400% dividend(Rs.8)
http://www.bseindia.com/corporates/ann.aspx?scrip=532331&dur=A&expandable=0
March 24, 2016
Ajanta Pharma USA Inc., a wholly owned subsidiary of Ajanta Pharma Ltd, announces today the launch of Levetiracetam Immediate-Release Tablets (250mg, 500mg, 750mg, 1000mg), a bioequivalent generic version of Keppra in the US market.
Levetiracetam is used for the treatment of various types of epilepsy.
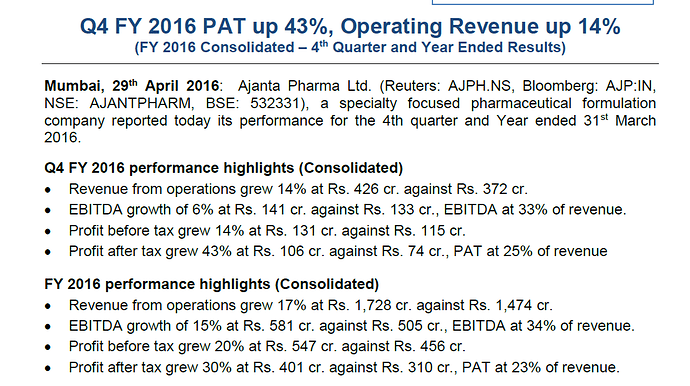
Ajanta Pharma announced its Q4FY16 results
https://www1.nseindia.com/corporate/AJANTPHARM_press_29042016132216.zip