Wockhardt’s novel antibiotic Zaynich has successfully treated a severe pan-drug resistant infection in a U.S. liver transplant patient.
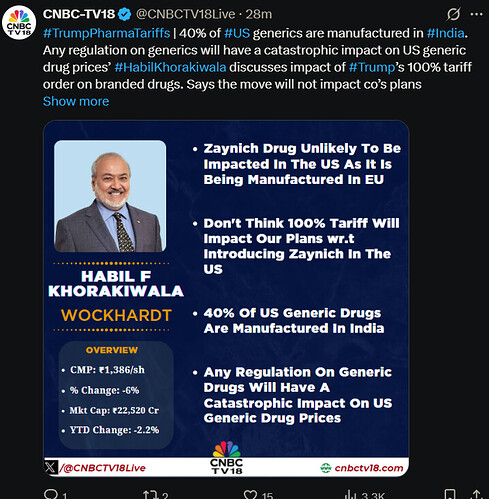
What is the repercussions of Trump pharma tariff on Zaynich? As the expected tariff may reach 250% in 2 years coinciding with launch of Zaynich, a damocles sword is hanging on Wockhardt share. Experts are requested to give insights.
Wockhardt would have patent and exclusive rights for it’s formulations, so I guess Wockhardt can bypass this by having it contract manufactured in US or license it to a US brand.
This may lead to erosion of some profit margin or may increase price for US.
Press Release - “Prestigious Medical and Public Health Journal " The Lancet Regional Health” Publishes Pivotal Phase 3 Clinical Study on Miqnaf (Nafithromycin)
https://www.thelancet.com/journals/lansea/article/piiS27723682(25)001374/fulltext
A phase III data on nafithromycin has been published.
Non inferiority against an antibiotic - Moxifloxacin has been established. (Not superiority)
Moxifloxacin became approved in 2010.
A 7 day course of Moxifloxacin costs about 175/-.
How much more patients will be willing to pay for a shorter three day course for comparative efficacy ?
Rgds
The reports says that
ECR was observed in 91.3% (220/241) of patients in nafithromycin group and 89.0% (210/236) of patients in moxifloxacin group of the MITT population [difference, 2.3%; 95% CI (−3.1, 7.8)]
So why nafithromycin was not superior if any one can explain
Wockhardt has filed a New Drug Application (NDA) with the USFDA early last week [Moneycontrol].
Following the NDA submission in October 2025, Zaynich will undergo the USFDA’s structured review process. The agency first conducts a 60-day filing review to determine if the application is complete. Once accepted, the drug may be granted either Priority Review (6-month timeline) or Standard Review (10-month timeline), culminating in a decision by the Prescription Drug User Fee Act (PDUFA). The process includes facility inspections, labeling negotiations, and potentially an advisory committee meeting, especially for novel therapies.
I am expecting Zaynich to be launched in the US before the end of calendar year 2026.
Its going to be fast track review…for approval…Good trigger
Zaynich Launch Timeline: -Total TAM- $9B globaly
India: End of FY25
Global: FY26
US: FY26 (May 26- Jul 26)
BEYOND ZAYNICH: THE PIPELINE Fast Track for Zaynich validates Wockhardt’s entire anti-infective platform:
ERTAPENEM-ZIDEBACTAM (WCK 6777) EMROK/EMROK O 4 novel drugs. All targeting critical resistance gaps.
So there are 4 more like this…Very close tracking required.