Thanks for bringing up this point. I agree when viewed this way, the compensation seems on the higher side but this is at the moment only an enabling resolution. We need to wait and see how much they actually take and what are the revenues at that point of time. Sakar’s promoter remuneration has been within normal range until now though it has increased rapidly in recent years. I view it as a negative but not a show stopper as yet. If the revenues increase in line with the salary, it would be okay I guess. But yes, this is a point that needs to be watched.
Hello guys,
i had two questions -
-
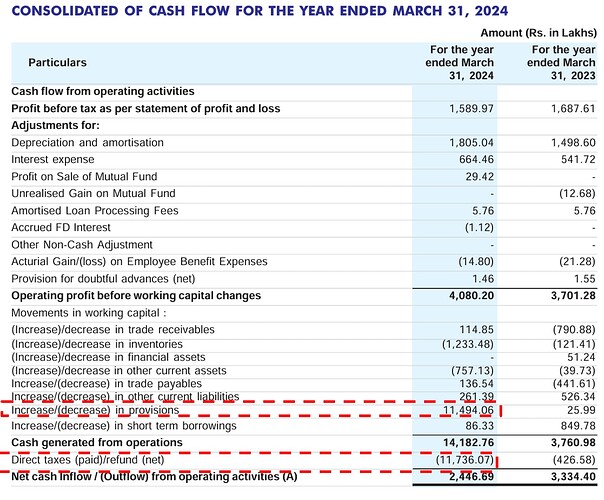
As per the AR 2024 cash flow statement they received ~Rs 115 crs via increase in provision and there was a tax cash outflow of Rs ~117 crs - can anyone clarify on this transaction as there is nothing in balance sheet provisions and notes - Attaching screenshot below for reference -
-
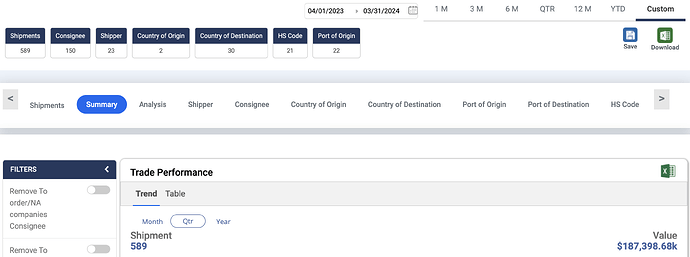
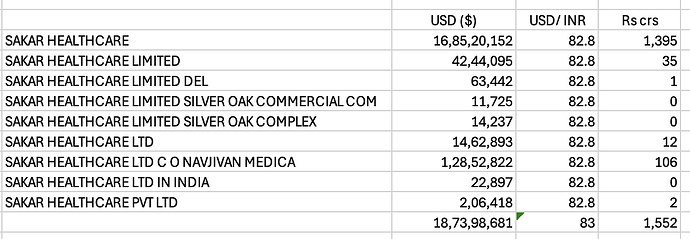
I was checking Sakar healthcare’s export data on Volza - as per company’s AR 2024 - they exported goods worth Rs 94 crs - but as per the volza portal the exports were to the tune of ~$ 187 mn which translates to ~Rs 1500 crs - there were six sakar healthcares with same address - couldnt find anyother company online which could also be named Sakar Healthcare and all these shipments source country was India - Anyone has any idea what is it that i am doing wrong here - Attaching screen shots of volza below for reference -
Thank You
Hi, On point no. 1, this is an error. There is some typo or something, I have got it confirmed from the company. While this is an error, the company has confirmed that the final figure of Net Cash Inflow is correct due to the offsetting impact of Increase / (decrease) in Provisions and Direct Taxes.
Nuvama Emerging Ideas 2024 Conference note on Sakar:
Sakar Presentation was today, This is Pre-Conf.
Sakar health care will supply 5 oral solids / 2 injections to accord healthcare for oncology products .
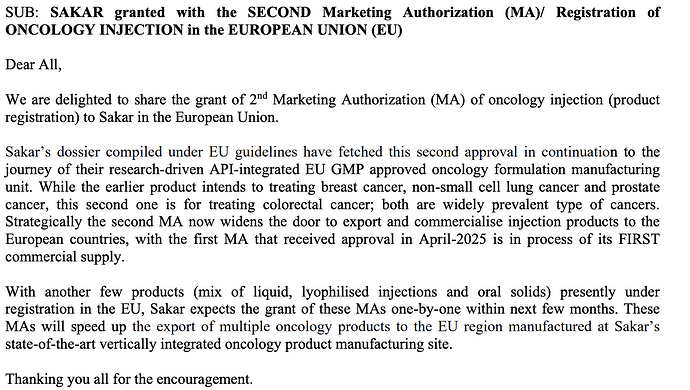
SAKAR granted with THIRD & FOURTH Marketing Authorization (MA)/ Registration of
ONCOLOGY INJECTIONS in EUROPE
There has been two more Marketing Authorisations (MA) granted with oncology injection (product registration) to Sakar in the European Union, taking the count to four MAs.
Two of the product dossiers for Carboplatin and Docetaxel compiled under EU guidelines have fetched approvals from Bulgaria and Bosnia. Both the dossiers are a part of Sakar’s present effort in regulatory front from its research-driven API-integrated EU GMP approved oncology formulation manufacturing unit.
While the earlier products (MAs) intends to treating breast cancer, non-small cell lung cancer, prostate cancer, colorectal cancer; Carboplatin is intended for ovarian, lung cancer along with other solid tumours.
Strategically these approvals now widens the scope to export anti-cancer injections to the European countries, while Sakar prepares the commercial supply with the first two received MAs.
With a mix of oncology products in process of registration in the EU and rest of world markets, Sakar expects to receive grant of more MAs within few months. These MAs will speed up the export of multiple oncology products to the EU region manufactured at Sakar’s state of the art, vertically integrated oncology product manufacturing site.