Which are listed Medical Devices players in India namely in Cardiovascular and Ortho fields ??
Cipla has some stake in device company. But unfortunately management control is with other gentleman who is not very gentle. So its probably dud for cipla.
Does anyone know the implications of “observations” made after a US FDA audit?
Disc: Heavily invested
Observations are the points where FDA has sought clarification from the company. The company has to send the reply on observations within stipulated time which is 15 to 20 days. On receipt of that response FDA further evaluates and give its comment.
Observations per se has no business impact. But if the response are not acceptable or to the satisfaction of fda they may issue warning. Warning letter will mean no new products can be manufactured at that site but existing business continues. Meanwhile company keep sending response to the observations to satisfy the authority. Generally it takes 6 months or more to reach the warning letter stage.
If these efforts also fail, then import alert is issued and existing business also stops. This takes few more months.
This is my understanding…
“During the inspection, our investigator observed discarded CGMP documents and evidence of uncontrolled shredding of documents. For example, multiple bags of uncontrolled CGMP documents with color coding indicating they were from drug production, quality, and laboratory operations were awaiting shredding. Our investigator also found a blue binder containing CGMP records, including batch records for U.S. drug products, discarded with other records in a 55-gallon drum in your scrap yard. CGMP documents in the binder were dated as recently as January 21, 2019: seven days before our inspection. Your QU did not review or check these documents prior to disposal.”
“In your response you state the binder of CGMP documents in your scrap yard was “inadvertently come [sic] to scrap yard” and that you were investigating the issue.”

worth a look … with a pinch of salt
Unlike small molecule generics, …biosimilars that are approved by the USFDA need to be promoted under brands and are not interchangeable for innovators’ brand prescriptions…the USFDA issued guidelines …have raised the entry barrier for biosimilar players…Unless biosimilar are interchangeable, they remain prone to competition from biobetters.
Indian pharma exports may grow by 15% and touch USD 22 billion during the current financial year against USD 19.14 billion in FY19.
The cumulative growth of Indian exports for the period April-July was 13 per cent. As of June 2019, India’s generic pharmaceutical exports have grown almost 2.7 to 2.8 times faster than the market (global generic market).
As exports have grown by 13% in first four months & expected to grow 15% YoY, it is a clear indication that the growth will pick up in balance period, which was already 21.7% in July.
This if implemented will be BIG for the domestic API manufacturers and also for the formulation companies if they can start sourcing from Indian API manufacturers since they will get out of the price control net.
Thane-based Generic Aadhaar is a pharma startup that provides generic quality medicines from reputed pharma companies at up to 80 percent lesser prices. It offers a huge portfolio of branded, generic, homoeopathy, and Ayurveda medicines from government-approved manufacturing facilities.
A startup like this, if sustainable, can spoil the life of branded players.
Mankind Pharma Ltd, India’s largest unlisted pharmaceutical firm, plans to launch an initial public offering (IPO) in two years, as its private equity (PE) partners want to offload their stakes, the company’s founder and chairman R.C. Juneja said.
Pharma is struggling for quite long and always a new issue specially from regulating agencies keep on popping up.
“The issue of unethical use of marketing tactics has been escalated to the Prime Minister’s Office (PMO) which, in turn, made calls to some top drug-makers and fixed a meeting with the PM. The PM told drug-makers that their non-compliance with marketing practices is pushing the government to create a strict law. He has warned about bringing in a statutory provision, and indicated that the ministry (of chemicals and fertilisers) has been asked to start working on it,” said a senior government official who attended the meeting.
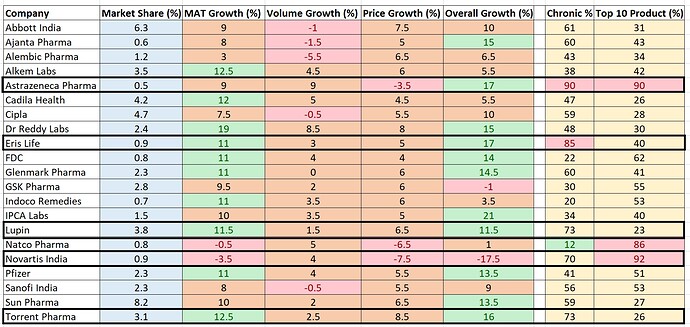
(Data source: Toshniwal Equity)
Many pharma companies have posted double digit growth(Domestic)
Due to present covid crisis hospitals are having less than 50% occupancy,elective procedures are postponed, many OPD clinics are closed or working for limited hours. Its obvious that overall medicine consumption will be down in India. I think domestic pharma growth will be hampered to reasonable extent.
I was looking at domestic companies which makes maximum revenue from chronic therapies which are to continued irrespective of present situation.
Going by above table AstraZeneca,Eris,Lupin,Novartis and Torrent gets more than 70% of revenue from chronic therapies. Their growth % much better compared to others for March month(except Novartis) possibly due to some pre-buying.
It will interesting to watch their sales no for April and subsequent months but de-growth is unlikely to happen in chronic therapies.
Discl: Invested: Astrazenaca and Ipca lab.
One quick question, Astrazenaca top 10 is contributing almost 90% (revenue?). Is not the product line too concentrated. As in, can it withstand competition, if any, to its product line ?
The products that Astra had launched were innovative products. One of their big products is Brilinta where they will be facing stiff competition in coming months due to loss in exclusivity/patent. They have launched 4 more innovative products in India with no competition and it has to be seen if they are able to ramp up those products. Product concentration is a risk no doubt about it .