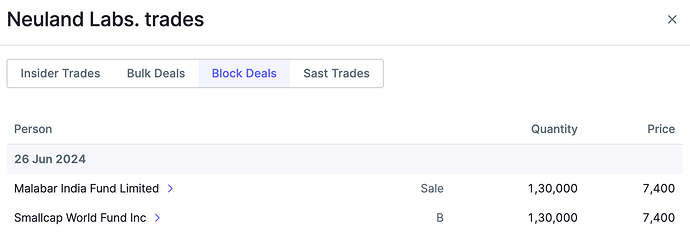
One more update on Neuland.
The latest annual report of Neuland Lab.
https://www.bseindia.com/xml-data/corpfiling/AttachLive/a330fda6-abd5-44fd-8860-a17ed8400e30.pdf
Below is the AGM notice. AGM is on 31st July
Does the change of Logo symbolizes the transition? Is the growth already priced? Mgnt said now in consolidation base , post FY25 onwards will see growth.
Sharing an in depth video on Pharma Value Chain by SOIC
Neuland is discussed in depths from 16 to 22 minutes on the time line.
Hope you find is useful
Invested and Biased
dr.vikas
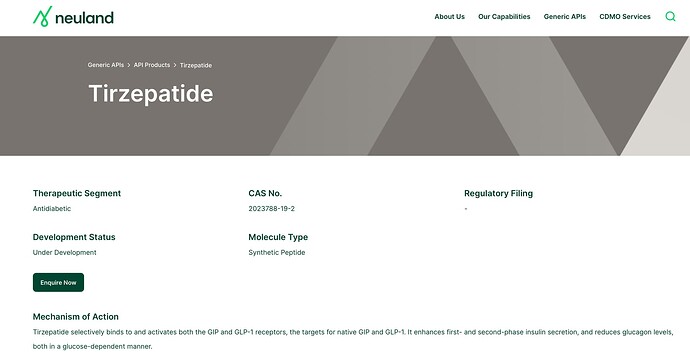
Sharing below a fascinating article on obesity drugs.
The Race for the Next Ozempic | WIRED.
Interestingly, Eli Lilly’s tirzepatide is more promising than the semaglutide. And Neuland is developing it.
Just sharing.
I am invested and biased.
dr.vikas
Neuland in the past concalls has referred to developing tirzepatide but its under patent till 2035. so how can neuland benefit from developing it even in forseeable future. plz clear my ignorance here. thanks
Aren’t Trizepatide and Semaglutide already commercial? Lilly is selling Trizepatide as Mounjaro/ Zepbound, and Novo selling Semaglutide as Ozempic/Wegovy.
Is Neuland helping Lilly with commercial manufacturing?
Maybe. Possible. I don’t know, frankly.
We have to ask them.
Excellent reults from Neuland.
I hope you find it useful.
Neuland Labs -
Broad brush highlights from management’s Q1 commentary -
Expecting moderate growth this FY. Growth in last two FY’s was exceptionally high. Won’t get repeated this FY. Management asked the audience to draw their own conclusion from this ( my deduction - going by the management’s tone, I guess they are hinting at 10-15 pc kind of growth rates for current FY - to be taken with a bucket of salt )
Also guided at pick up in growth rates in FY 26
BioSecure act should be a huge tailwind going forward for the CDMO Industry. Seeing increased customer visits as a lead indicator of customer’s interests wrt Indian players
Traditionally, Neuland did most of its innovation related work with smaller Bio-Tech companies. As these companies are being acquired by big Pharma companies, Neuland hopes to become a household name with big Pharma as well ( in next 3-4 yrs )
CDMO Pipeline -
Current number of molecules in Phase 3 @ 07 ( 03 APIs + 04 Intermediates )
No of commercial molecules @ 18 ( 08 APIs + 10 Intermediates )
No of molecules in pre-commercial stage @ 14 ( 08 APIs + 06 Intermediates )
Disc: holding from lower levels, biased, my largest portfolio position, not SEBI registered, not a buy / sell recommendation
Holding neuland labs since 2021 , A quick update on Q1FY25 result
Financial Overview (Q1 FY25)
Total Revenue: ₹444.4 crores, up 21.7% from ₹365 crores in Q1 FY24.
EBITDA: ₹128.6 crores (excluding exceptional items), with a margin of 28.9%, an increase of 174 bps from Q1 FY24.
Gross Margin: 56.1%, compared to 55.2% in Q1 FY24 and 58.8% in Q4 FY24.
Profit After Tax (PAT): ₹98.3 crores, compared to ₹62.2 crores in Q1 FY24. This includes an exceptional gain of ₹30.6 crores from the sale of surplus properties.
Earnings Per Share (EPS): ₹76.6 per share.
Free Cash Flow: ₹50.9 crores generated in Q1 FY25.
Net Debt: Negative net debt position of ₹110.2 crores, with partial debt repayment of ₹8.7 crores.
Capital Expenditure: ₹59.1 crores invested in Q1 FY25.
Segment Performance
Contract Manufacturing Services (CMS)
Revenue Characteristics: ₹235 crores in Q1 FY25; variability in quarterly revenue. Annual trends are a better indicator of performance.
Revenue Drivers: Commercialization of key molecules, influencing lumpiness in revenue.
Generic Drug Substances (GDS) and Specialty APIs
Specialty APIs: Dorzolamide and Donepezil: Niche therapeutic areas with strong performance.
Prime Products: Key Products: Mirtazapine, levetiracetam, escitalopram, providing stable revenue.
Ezetimide Transition: Transition from specialty to prime segment affects specialty segment performance.
Strategic Outlook
FY25 Expectations
Growth and Margins: Modest growth anticipated, with normalized margins. Focus on operational efficiency and consolidating investments.
Impact of Investments: Ongoing capital investments and product lifecycle may impact revenue growth.
Future Growth (FY26 and Beyond)
Manufacturing Facilities: New facilities and commercialization of new molecules expected to drive growth.
R&D Pipeline: Strong pipeline with promising molecules in development.
Macro Environment
BioSecure Act: Limited immediate impact but optimistic for long-term prospects.
Big Pharma Acquisitions: Opportunities and challenges as biotech clients are acquired by large pharmaceutical companies.
Medium and Long-term Prospects
Order Flow and Business Confidence
Order Flow: Strong order flow with confidence in medium and long-term prospects.
Influencing Factors: Product performance, foreign exchange rates, and raw material costs.
Capacity Expansion and Production Plans
Unit 3: Timeline: Expected operational by end of FY25, with commercial production starting H2 FY26.
Capacity Utilization: Initially at 30-40%; expected to increase as more CMS molecules are integrated.
Units 1 and 2: Current Utilization: Near full capacity; acquired land for future expansion.
Short-Term Capex: Small additions to meet increased demand for GBS products.
.
Specialty APIs Market Opportunities
Market Data
Pelivaradon: Limited competition, niche market.
Penta Capone: Moderate competition, with Indian and European players.
Dozen Lite and Apex Bar: Market competition varies by therapeutic demand.
Capital Utilization and ROA
Unit 3 Utilization: 30-40% in Q1 FY25, expected to rise with increased CMS molecules.
Cost Reduction: Reduced manufacturing expenses by 30-40%.
Historical ROA: 10-15% for similar expansions; future ROA dependent on market conditions.
Customer and Market Trends
Customer Expansion
Current Clients: Increased engagement from existing biotech clients bringing additional molecules.
New vs. Existing: Growth from both new and returning clients.
Biotech to Pharma Transition
Focus Shift: Biotech companies being acquired by larger pharma companies; potential for expanded collaborations.
Future Plans and Vision (5-Year Outlook)
Current Focus
API Model: Continued success with PurePlay API model, significant growth potential.
Market Opportunity: API market valued over $120 billion; company still in early stages of market capture.
Future Strategy
Growth: Focus on API model for GBS and CMS.
Diversification: Explore new technologies and innovations within the API space.
Objectives: Enhance drug development and manufacturing processes.
Risk Management
Primary Risks
Supply Chain Issues: Reducing dependence on Chinese suppliers; exploring alternative sources.
Geopolitical Risks: Impact from events like the Russia-Ukraine conflict.
Currency Risks: Monitoring fluctuations as part of risk management.
Stock Split Consideration
Board Discussions
Consideration: The stock split idea under review; discussions ongoing with advisors to determine feasibility.
Normalization of Margins
Past and Current Guidance
Historical Margins: Historically 14-15%, with peaks around 28-30%.
Normalization: Aiming to normalize margins without specific past performance references.
Factors Influencing Margins
Business Model Shift: Reduction in reliance on prime products affecting margins.
External Factors: Variations in raw material costs and exchange rates.
11. New Peptide Molecules and Pipeline
Peptide Development
Chronic Kidney Disorders: New peptide molecule in development.
New Developments: Six to seven new molecules in various stages, with a focus on specialty areas.
Specialty Focus: Many new molecules have longer development cycles.
Overall long term view for Neuland :
-
Company is net debt free (long term as well as short term) as it is having casah more than borrowings.
-
Company is generating around 75 Cr free cash flow every quarter.
-
The free cash flow generated by the Company is sufficient to take care of maintenance capex as well as growth CAPEX.
-
Management has proven its integrity and is having a very good sense of efficient Capital allocation.
-
Management track record is of being very conservative/efficient for CAPEX.
-
Management had historically been conservative in giving future guidance.
-
Business has over a period of time has evolved for high margin business.
-
Management has a very focussed approach and clarity about future business.
-
Management is constantly evolving itself to adapt to the new business enviorment.
-
Managment is well aware of the various kind of risk involved in the business and is constantly monitoring and reviewing the same.
-
There is a sector tailwind and company is well positioned and adequately capitalized and leveraged to take advantage of the same. Company is well positioned to fund its growth from all means internal accruals, Equity, Debt. Interanl accrauls are sufficient to take care of 15 to 20% growth for 5 to 6 years.
-
CDMO sector has a very long run way and visibility for at least 10 years.
-
Track record of the Company has given it a recognition in the CDMO sector and this is going to further evolve and improve in coming years.
-
Commercialization of some key molecules in next 12 to 24 months will further establish the brand image of Neuland and more molecules may come for development from existing as well as new molecules.
-
Approval and commercialization of block buster drug KARXT in Sept 24 will be a big booster for the Company both in terms of revenue as well as establishing its credibility in the CDMO sector.
-
Neuland has been associated with Karuna Theuraptics for development of KARXT drug. Acquisitinon of Karuna Therauptics by Bristol Myer was mainly for the single drug KARXT. Bristol Mayer is very big name in big pharma and going forward in next 5 years , we can see sourcing of development of new molecules to Neuland not only from small Biotech Companies but also from big pharma. Market cap of Bristol Myer is around USD 100 Billion.
-
DII holding is very less at around 6.39 % and will give support during any major downfall.
-
Succession issue is not there for at least next 10 to 15 years as current generation Suchet and Saharsh are young and very efficiently handling the business.
-
Credit rating is further expected to improve.
19 Working capital cycle is further expected to improve.
-
Margin profile has significantly improved due higher contribution from CMS business and is expected to remain in the range of 20 to 25%.
Capacity utilisation is further expected to improve leading to triggering of operational leverage. -
Disclosure standard is best in the industry and Corporate governance track record is strong.
Disclosure: Invested and views are fully biased. Holding for last 3 years and will hold for next 5 years from here.
The long-term outlook for Neuland appears highly promising, as the company plans to commence utilization of its third plant by the last quarter of 2025, initially operating at 20 to 30% capacity for CMS , which is expected to significantly boost its production capabilities and growth prospects.
Neuland they are saying in the article, will not benefit as much as some other CMO and CDMO companies like Syngene Int., piramal pharma, and Lauras Labs as Neuland is mainly into APIs…
Analyst/CNBC Anchor are wrong regarding Neuland as they are Dedicated API player and not into formulation so IP violation chance is less and innovator more happy to work with Neuland. In fact cautious tone of management is taken as warning by people who have not followed Neuland management for long…
How strong is Neuland is in biological CDMO space.
JUDGEMENT DAY FOR NEULAND ON 26.09.2024 as FDA may approve block buster drug KARXT.