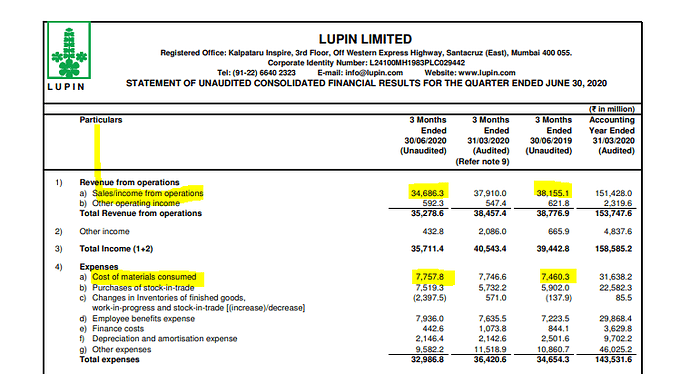
Lupin concall:
The management is very confident for complex generic portfolio for next few years and believe they are at inflection point.
Lupin hope to get approval for proair by August and a launch by September.
Hope to relaunch Metformin by end of Q2. They don’t see Rx to change to alternative products as still few products are there in market without NDMA issues.
US business to do better on QoQ basis…
India business to grow by double digit this financial year.
Solasec demand coming back slowly.
Prescription to improve substantially going forward.
Expecting Etanercept launch in Europe this quarter. They would launch in Germany via partner this month only.
Fostair Europe launch also expected in this Financial year.
Goa, Pithampur and Somerset re inspection should happen in next 3 months.
Albutrol pricing likely to happen @20-30 pc discount and it would lead to higher competition amongst existing players.
US business is lower largely due to early storage in March and low sales of sporins and influenza products…
Management sees not much impact of trump order.
Employee, sga expenses to be lower as pc of sales going forward.
Maintaining r&d to be lower than 10 pc of sales in long term.
Management is looking for ebidta margins in early twenties going forward.
Can touch USD 200mn back by Q3 this year.
Partnership with Fordoz is for 2 molecules in injectable portfolio. Impact likely to be seen in next 2 years.