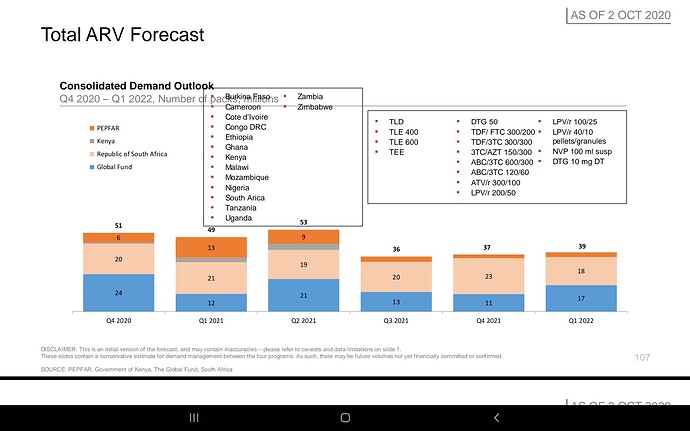
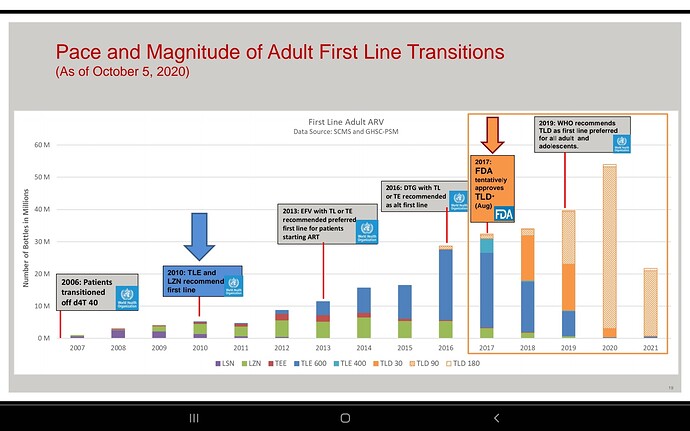
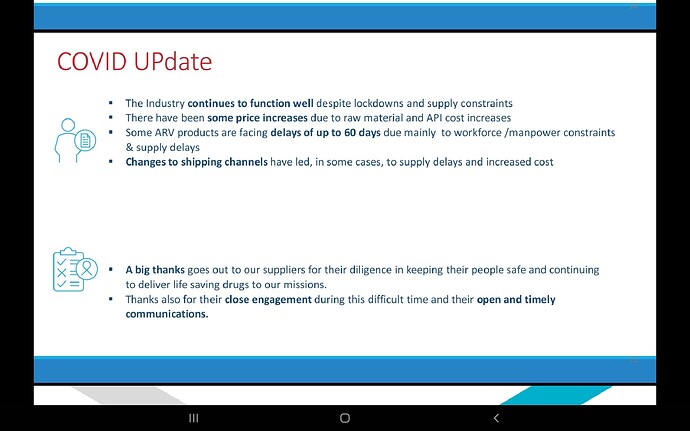
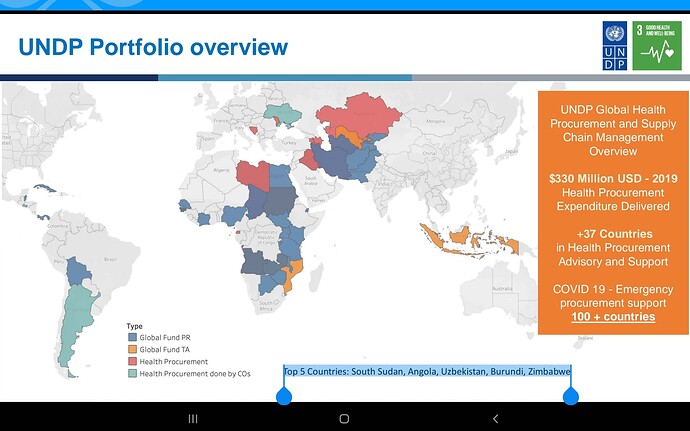
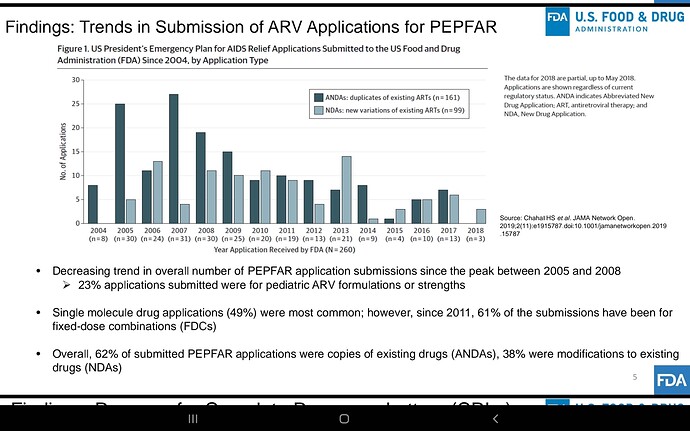
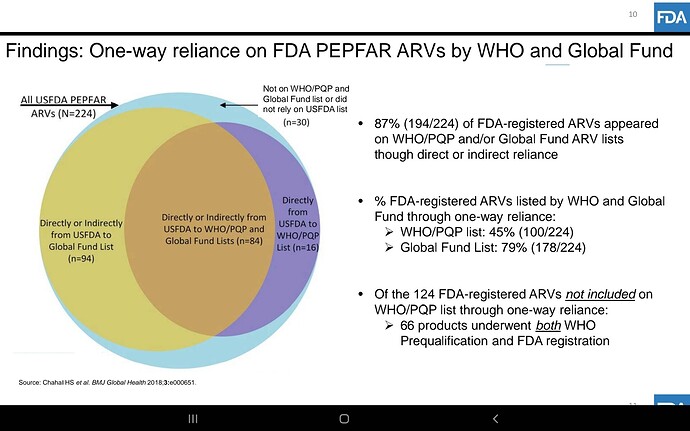
Companies will compete but Feel the market here is massive infact the whole world… Don’t know about others but Laurus has advantage due to its close proximity with MPP, very good experience in ARV , low costs, can scale up massively very quickly due to its huge ongoing expansion, if required can be diverted to this, so hoping for the best😊
Just in June there was a this news…
Laurus Labs Ltd is developing a third-line HIV treatment, darunavir boosted with RITONAVIR (DRV/r), for children.
The development is under way based on an agreement inked in June 2021 by the Hyderabad-based company, Unitaid and the Clinton Health Access Initiative, so world knows Capabilities of Laurus in this field.
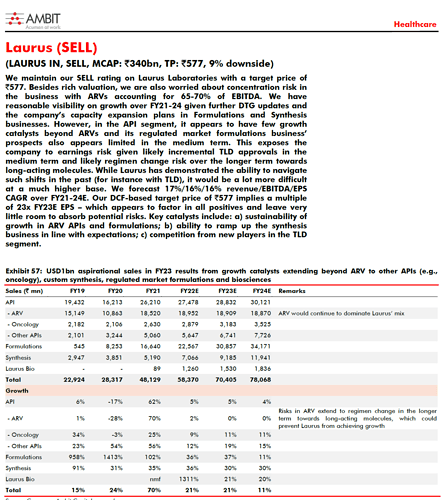
Jeevan patwa a expert had tweeted & i agree …Majority of 1700 crs capex will commercialize by Mar-22 which is into non-ARV and generate roughly 2500 crs. This capex is already tied up for clients, so capacity utilization will happen from Q1fy 23 itself
The sales from COVID pills will be the huge icing on the cake when order received , i think , COVID or No COVID Laurus will march ahead, again in my view this is a decadal theme the only concern which i forgot to mention last time I feel is …execution, as their plate is full , strategy is in place, hope if required they will keep hiring the right managers…, To manage all this skillfully But was somewhat relieved after reading this news  Vivek Digumarti IT head from @LaurusLabs was named a winner at the #IDCIIA2021 in the #Innovation in #Operations category for the initiative to conduct #virtual facility tours by using #realwear Smart glass.
Vivek Digumarti IT head from @LaurusLabs was named a winner at the #IDCIIA2021 in the #Innovation in #Operations category for the initiative to conduct #virtual facility tours by using #realwear Smart glass.
Event: http://bit.ly/3s4CAhF I Winners: http://bit.ly/3GKNOvE! News by IDC India
thusThey are taking some steps towards very efficient execution thankfully, also for succession his son Mr. Krishna Chaitanya spearheads the Synthesis and Ingredients divisions of the Company and has rich work experience in strategy, & is highly educated thus is being groomed by his father
Corporate governence i don’t think is the issue here Cause if you read the reason given in the article why they were chosen in top 5 finalist out of so many companies for corporate governance award by moneylife foundation its very overwhelming… These are just my thoughts thinking aloud with you friends not good in accounts so number crunching is not my forte… Just do back of the envelope calculation, can be wrong please guide take care stay safe.


 . When you are in love with the company all you find is confirming evidences, which is one of the biggest enemies of investing.
. When you are in love with the company all you find is confirming evidences, which is one of the biggest enemies of investing.