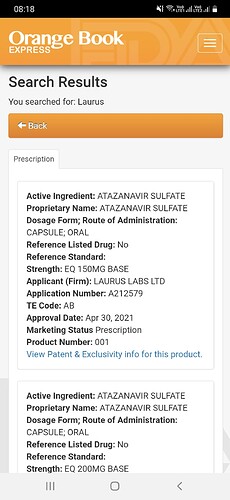
All the drugs of Laurus lab are still in active prescription list, including Atazanavir sulphate, as of today, with AB rating in Orange book. As per the announcement from FDA, it already has been changed the therapeutic equivalence (TE) rating for all the affected generic products involved in Panexcell/ Synchron to “BX”. If FDA is to be believed, then none of the products of Laurus lab are involved in this controversy so far.
As per EMA document it is not 18 products of Laurus lab, but a single drug Atazanavir 150/ 200/ 300 mg capsules in 6 countries of Europe, as of now even that issue is resolved.
Laurus lab got approval for the same drug from FDA only in April 30 - 2021.
Atazanavir is not a first line of prescription drug, not even second line main treatment option. It is a additive drug for second line of treatment and for prophylactic use.
This issue was communicated to Laurus lab by FDA in March 2021, company taken necessary actions ( if sources are true) and approval came in April 30 - 2021 ( after this whole fiasco).
As of now FDA related contribution of this single product ( after April 30 - 2021) to the total sales is negligible, even if it is going to be affected it will be for short term ( since corrective measures are already initiated).
Since this information is there in public domain from September 15 -2021, possibly the unfounded fear might be the reason ( or just a coincidence!) for the negative price action from consolidation range. Hopefully it is already factored in and digested. On the other hand it is a good news for better pricing of generic drugs due to shortage.
This topic is temporarily closed for at least 4 hours due to a large number of community flags.
This topic was automatically opened after 20 hours.
still it is being shown as AB, seems laurus labs hasn’t used services of any of the banned CRO for filing the ANDA.
Laurus products are still in AB TE code.
Then the other thesis can be the continued delay of container which they said in previous qtr that the shipment delayes will be cleared in q2.
As per Share Khan report on 29th Sep 2021 recent regulatory Concerns are Overdone and growth prospects are intact. The report says after management interaction, the management has clarified on this news and has stated that just one of its products
had been impacted and that has a negligible contribution to the overall sales. The other
products are in the prescription list and would continue to grow, thus pointing at concerns of being overdone. Therefore, the correction in stock price has been steeper than the concerns and now provides for a good entry point for investors.
It’s a free report any one can access @
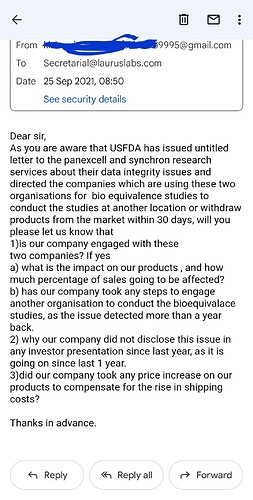
Laurus labs management is being unethical by disclosing this kind of price sensitive information to select people. This is bad corporate governance, I wrote to their investors relations team on 25th of September regarding the usfda issue and still they haven’t replied. But sharekhan got this info and they might have used it to their advantage.
They will only make a disclosure about something if there is a material impact on business.
Panexcell and Synchron CRO issue had nil to miniscule impact on business, hence they disclosed nothing.
Just because a set of retail investors misinterpreted some news, assumed the worst and sold their shares, is it the company’s mistake?
This entire episode teaches one to think about for every company news if an investor reacts, gets in and out of a stock he cannot make anything. There can be many hurdles ahead but in my view a long term investor should leave these day today operations/problems to the management to handle and whose focus on long term wealth creation. As this issue do not have any material impact, may be management didn’t want to publish.If we go behind every company to find some fault, not at all possible to find one that’s 100% good same as that of an individual.
Disc. Above views are personal. & Invested.
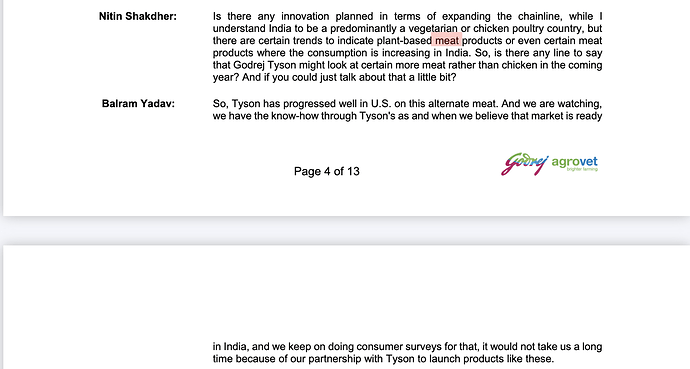
Nirmal bang has published a report of alternative protein market . animal cell based protien expected to grow at cagr of 66% from 2025-30 . A good read
Disc : I hope this report can be shared as it can be publicly accessible without any login
I did quick search for plant based meat and found below results.
Hindustan Unilever Limited(Integrated Annual Report 2020-21)
Tasty Bites( 36th Annual Report 2019-2020)
ADF Foods(Anual Report 2020-21 P.No- 65)
Godrej agrovet
From AR of 2020 - 2021 of Laurus lab
“Laurus Bio is into Biotech space producing Enzymes, non-animal cultured meat etc.”
So how big this opportunity and how Laurus can get benefited from this? can any one help me to understand this.
i am new to investment journey.
According to MarketsandMarkets, the plant-based meat market is estimated to be valued at USD 4.3 billion in 2020 and is projected to reach USD 8.3 billion by 2025, recording a CAGR of 14.0%, in terms of value. It is witnessing significant growth due to growing vegan and flexitarian population across the world, rising awareness about the health benefits offered by plant-based meat over animal meat, and growth in government initiatives along with significant investments are driving the global market.
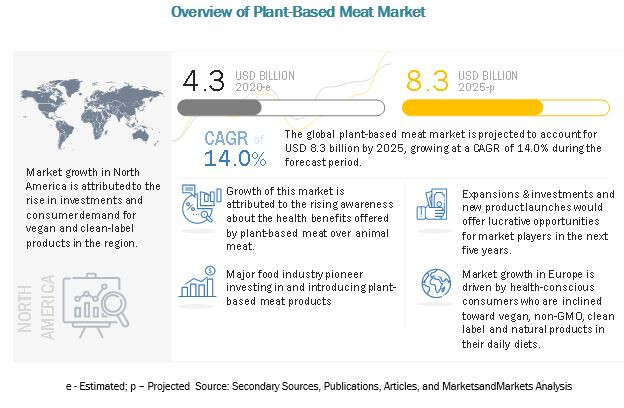
Reference - https://www.marketsandmarkets.com/Market-Reports/plant-based-meat-market-44922705.html
Someone please double click on what could be contribution by laurus in this space and what is their competitive advantage if any in this space?
Lab-Grown meat & Plant-based meat are two different things.
Laurus Labs is into lab-grown meat which is made using animal cells & Recombinants proteins in bioreactors.
Recombinants & Fermentation (Bioreactors) is what Laurus Bio will be involved in by making Non-Pharma Grade recombinants & building fermentation capacity.
Management’s latest communication on prospects
- Laurus Bio could contribute meaningfully from FY23.
- No meaningful impact of CRO problem on their numbers
- Non ARV APIs will recover in 2QFY22
- ARV APIs will normalize in 3QFY22
- Not worried on ARV pricing as cost reduction and volume growth can make up for the same.
Disclosure: Invested
Could someone clarify whether laurus makes Molnupiravir API?
Source-VSEZ fastracks approval for production of Remdesivir - The Hindu
Here is the part of Q1 FY 22 conference call, where there was mentioning of Molnupiravir……
Bharat K Siripurapu ( Question):
In the recent interview with one of the international media channels, when you are asked one question on what excites you in future in Laurus, you said that you expect there would be an oral drug for COVID, and if it comes it will be a big opportunity for Laurus. May I know if there is any COVID oral drug in pipeline like Molnupiravir with Laurus?
Dr.S Chava ( answer):
As you are aware, we in-licensed the two DEOXY-D- Glucose from DRDO, for which we are gearing up for launch in the next few weeks. We’re in the regulatory approval process. And other than that, we are working on other drugs, but we don’t know when the approvals come. It’s too early to predict.
Molnupiravir API
According to Mr. Rama Mohan Reddy, the Laurus labs of the APSEZ has supplied 38.3 million HCQ tablets during the first wave of the pandemic last year to various countries such as South Africa, Singapore, Burma, Belgium, Canada and USA. It is now in the planning stage to manufacture Molnupiravir.
He also said that the VSEZ had given approvals for manufacture of Molnupiravir in API form to the Honour Labs at the Nakkapalli SEZ, to the tune of 100 Kg/annum on a war-footing and is already in the process of manufacturing.