Collective Management Commentary : My Notes on Gufic
Gufic Business Structure
Industry Structure as of 2022 :
• Botulinum Toxin Indian Market Size is 120 Crore at the moment (Potential is huge in India and international market as well but it is not easy)
• Botulinum Toxin International Market Size 8 Billion and USA 5 Billion
• Critical Care India Market size is 181000 Crores in India
• Infertility is around close to 4000 crores in India and growing 15% yoy
• Dual Chamber Bag initially targeting Anti-infectives. Addressable market size is ~Rs. 3,000 crores in India
• Dydrogesterone product size for this product is ~Rs. 700 crores growing at 60% YoY in India
• Critical Care around 36000 Crores to 38000 Crores
• D29 Market size 6 million in India
Market Share
• Gufic has selected market segment in Critical Care around 36000 Crores to 38000 Crores out of which Gufic has products of 14000 to 15000 crore.
• Criticare they have 4% market share which is Rs. 200 crore sales in FY20-22.
• Ferticare, they have 3% market share which is around 120 crore
Company’s Standpoint - This might infrequent and change to market conditions
• we are the number 1 company for some Fungins
• we are the number 2 company for some antibiotics
• we are the number 3 company and because of these individual molecule markets,
• we already might be in double digit right now itself, we might be in certain cases maybe 20% of the market, in certain cases we might be 33% of the market share also
Merger/Partnership
• Gufic has also tied up with Metaverso TS, Portugal to launch series of recombinant molecules in India starting with the candidate ReF in the field of infertility. This will be the first recombinant product which will be made in Gufic’s facility.
• Gufic has tied up with Prime Bio, USA for manufacturing Botulinum Toxin API and formulation and they will develop several innovative formulations with Botulinum toxin in the field Dermatology, Neurology and Pain Management
Therapy Area: Toxins Strain transfer, Tech transfer, formulation development and manufacturing at Gufic
• Techno flex European leader in IV drug delivery systems. Collaborated with Gufic to launch Dual Chamber Bags for the 1st time in India for anti - infectives
• Bright Gene, China Therapy Area: Recombinant products and Anti Infectives Collaboration on several API to develop new product
• Cinna Gen Therapy Area: Infertility Tech transfer and Clinical development(Phase III) of the product at Gufic
• Lucas Meyer Cosmetics Therapy Area: Dermo Cosmetics Technical collaboration and Product Development
• Forayed into cancer immunology by undertaking research, collaboration with Selvax which is a research company based in Australia and as a part of this initiative is to focus on development activities in return for exclusive commercial rights for the immunotherapy in India along with an equal share of revenues from Europe and Selvax goal is to develop a very safe, effective immunological based treatment for a range of hard-to-treat solid tumours.
• GUFIC UK LIMITED (“GUL”), a wholly owned subsidiary of the Company, was incorporated in United Kingdom on March 15, 2022 with the intent of expanding the Company’s business in United Kingdom
| Partner | Therapy area | Type |
|---|---|---|
| Prime Bio USA | Toxins and Biologicals | Technical collaboration, Development, Manufacturing, Formulation |
| Pharmaaz | Emergency Medicine | Technical collaboration |
| Lucas Meyer | Derma-cosmetic | Technical collaboration & Product Development |
| BrightGene | Recombinant and Anti-Infective | Technical collaboration |
| Cinna Gen | Infertility | Technical collaboration & Clinical Development |
| Techno flex | Dual Chamber Bags | Technical collaboration |
| GUFIC UK LIMITED | Expansion into UK | Tie |
| Selvax Private Limited | Oncology | Tie |
Applications
| Salt Composition | Product |
|---|---|
| Omadacycline Tosylate | |
| Isavuconazole Sulfate | |
| Anidulafungin | Canidula |
| Caspofungin Acetate | Guficap/ Guficap Plus |
| Clarithromycin | Clarific |
| Colistimethate Sodium | Guficol/Guficol Plus |
| Doxycycline | Doxific |
| Fosfomycin | Fosfocide |
| Micafungin | Micafung/Micafung |
| Minocycline | Mific/Mific Plus |
| Paracetamol | Mol Plus |
| Polymyxin B | Polyfic/Polyfic Plus |
| Teicoplanin | Ticofic/Ticofic |
| Tigecycline | Tigefic |
| Ulinastation | UNlinafic |
| Vancomycin | Gufivan |
| Voriconazole | Vorific |
| Tranexamic acid | Tranafic |
| Methy! Prednisolone Sadium Succinate | Gufipress |
| Highly Purified Human Menotropin | Puregraf |
| Highly Purified Urofolitropin | Follicare |
| Human chorionic Gonadotrohins | Puretrig |
| Liposomal Emulsion Ampbhotericine B Gufisome | Emphoter |
| Vecuronium Bromide | Gufivec |
| Thymosin Alpha-1 | Alpha |
| Azithromycin | AZ-OD |
| Leuprolide Acetate | Luprocare Depot |
| Cetrorelix acetate | Cetrofirst/CBTROCARE |
| Enoxaparin Sodium | Gufinox |
| Botulium Toxin | Stunnox |
| Isoxsuprine Hydrochloride | TOCOLYTE |
| Piperacillin + Tazobactam | Tazofic/Tazofic Plus |
| Meropenem | Merofic/Merofic Plus |
| Dortpenem | Donific/Dorific Plus |
| Imipenem + | Imefic/Imefic Plus |
| Human Normal Alburnin | Albusure |
| Tab.Cefpodoxime | Prodox |
| Filgrastim | Gofigrast |
| Tab.Nitrofurantoin | Tab. UT Guard |
| Cefoperazone+salbactum | Cepofic |
| Posaconazole | POSAFUNG |
| Natural Micronized Progesterone | Gufigest |
| Human Normal! Immunoglobulin | Immunccin/Immunocin |
| Estradiol Valerate | Gufistra |
| Octreotide | Gufioctre |
| Dalbavancin Hydrochloride | GufiDal |
| Daptomycin | Gufidapt |
| Amphotericine emulsion | Ampofic |
| Dydrogesterone | Dydrofic |
| Estrodiol | Estafert |
| Loperamide | Ridol |
| Clotrimazole % + Neomycin + Beclomethasone | Lotril |
Gufic Biosciences Herbal Products
» Sallaki 400/600 mg Tablets ( Strengthens joints to cope with muscular pains )
» Sallaki M.R Tablets ( Muscle relaxnt )
» Sallaki Liniment ( Joint pains and inflammation )
» Laxive Powder ( The complete laxative )
» Imunocin Syrup ( Stimulates underdeveloped immune system )
» Livpar Tablets ( Fights liver damage )
» Aswal Plus Capsules ( The Stree Eleminator )
» Zulcer Capsules ( Combats hyperacidity, gastritis & chronic functional dyspepsia )
» Eugynin Tablets ( Gives freedom from menacing menstrual excesses )
» Eugynin-L Tablets ( Puts stop to leucorrhoea )
» Sallaki Plus Tablets ( Strengthens joints to cope with muscular pains )
» Sallaki Ointment ( Strengthens joints to cope with muscular pains )
» Nucart OA Tablets ( Management of Osteoarthritis )
» Rumastal Forte Tablets ( Fights aches and pains associated with fever)
» Imunocin Tablets ( Stimulates underdeveloped immune system)
» Livpar Syrup ( Liver tonic par excellence )
» Kofend Cough Syrup ( Relieves cough )
» Zulcer Syrup ( Combats hyperacidity, gastritis & chronic functional dyspepsia )
» Eugynin Syrup (Gives freedom from menacing menstrual excesses )
» StretchNil Lotion ( for Prevention of Pregnancy Stretch Marks )
Contracts
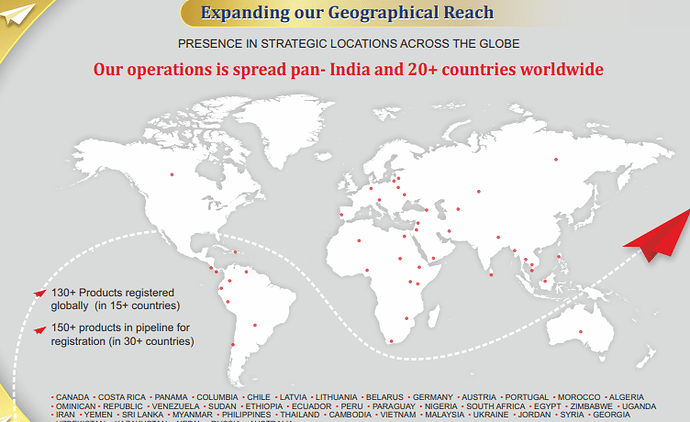
• 120+ Products registered globally on the name of Gufic in more than 20 countries
• 3 Contracts out of which two of them already the dossier is in place and going very aggressively, one might depend on the timelines of when will the Indore commercialization happen and time for the ANDA filing by the client.
Key Markets
§ Sri Lanka
§ Myanmar
§ Philippines
§ Vietnam
§ Canada
Key Products
§ Tigecycline
§ Liposomal Lyo Amphotericin-B
§ Micafungin
§ Anidulafungin
§ Vancomycin
§ Pantoprazole
§ Sallaki
Key Domination/Largest Supplier of formulations :
§ Doxycycline
§ Tigecycline
§ Gonadotropins
§ Liposomal Lyo Amphotericin B
§ Micafungin
§ Remdesivir (It has recently entered into an agreement with Hetero to supply Remdesivir – the only approved COVID-19 drug by US FDA. Hetero owns the license to market Remdesivir in 127 countries including India)
API & Intermediates Applications
ANTIFUNGALS
• Miconazole Miconazole Nitrate USP/BP/EP
• Cespelungin Acetate
• Econazole/Econazole Nitrate USP/BP/EP
• Micelungin Isaconazole Nitrala BP/EP
• Anidulafungin Ketoconazole USP/BP/EP
• Butoane.role Oxiconazole Nitrate (IHS)
• Terconazole Nitrate USP/BP/EP
• Tioconazole BP/EP
• Sulconazole Nitrale USP
ANTIBIOTIC
• Arbekan
ANAESTHETICS
• Prilocaine / Prilocine HCL USP/BP/EP
• Tetracine HCL EP/BP/USP
• Lidoraine / Lidocaine HCL USP/BP/EP
• Articaine HCL BP
INTERMEDIATES FOR ANTIFUNGALS
• 1-(2,4-Dichlorophenyl)-2-1 – Imidazole) - ethanol
UNDER DEVELOPMENT : ANTIFUNGALS
• Loliconazole
• Sertoconazole
Capex
• CMO have expanded the capacity pre-COVID of 31 crore
• 2021 Q3 and Q4 they have very aggressively invested in a new biological technology platform
• Indore Plant supposed to be Live on September as directed by Management
• Had some small expansion coming up for not only replacement but an injection of capacity of lyophilization
• Total capex of around 220 Crores for Indore plant
○ Out of 220 Crores 60 Crores has been already spent up to March 2022
○ 200 Crores for lyophilization facility including construction and land cost
○ Around 3 Crores to 4 Crores spent on the capex for our SAP implementation plus hardware for IT department
○ Indore plant 20 Crores they are spending for R&D facility
○ Remaining balance 160 Crores they are expecting to be incurred in the next financial year 2023-24
○ They are expecting internal accrual and for remaining amount
○ They are going to take a certain bank loan on a long-term basis.
• Around 25 Crores they have spent in Navsari plant for
○ Penem
○ Dual chamber
○ Increasing in lyophilization capacity.
• Gufic has invested in the development of H 15 - a candidate for Asthma and 3 new NDDS formulations for Anti-infective use.
Capacity Utilization
• 70% in lyophilization right now which should pick up
• 2.5 to 3 years window the company aims to 80% in capacity
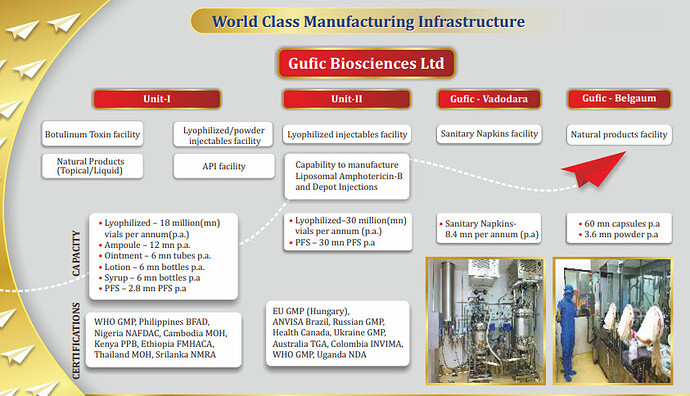
**• Unit - I at Navsari**
Lyophilized/Powder Injectables Facility : Natural Products (Topical/Liquid) API Facility Capacities
○ Lyophilized – 18 mn vials p.a.
○ Ampoule – 12mn p.a.
○ Ointment – 6mn tubes p.a.
○ Lotion – 6mn bottles p.a.
○ Syrup – 6mn bottles p.a.
○ PFS – 2.8mn PFS p.a.
**• Unit - II at Navsari : Lyophilized Injectables Facility Capability to manufacture**
○ Liposomal Amphotericin B
○ Depot Injections Capacities
§ Lyophilized – 30mn vials p.a.
§ PFS – 30mn PFS p.a.
**• Gufic - Belgaum Natural Products Facility Capacities**
○ 60mn capsules p.a.
○ 3.6mn powder p.a.
Upcoming
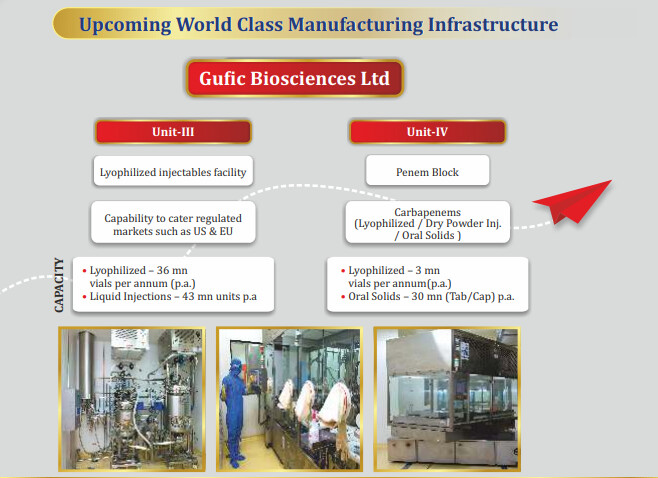
○ Unit - III at Indore Lyophilized/Powder Injectables Facility Capacities
○ Lyophilized – 36 mn vials p.a.
○ PFS – 15mn PFS p.a.
○ Liquid Injections – 60mn units p.a
**• Penem Block :** Dedicated facility for Penem Carbapenems
○ Lyophilized – 3mn vials p.a.
○ Dry Powder Inj - 30 mn Vials
○ Dual Chamber Bags-24 mn IV bags
**• UPDATE ON CAPEX**
○ Indore Civil Construction and Site Development work is progressing as per schedule and is near completion All equipment have been selected and orders have been placed and we expect it to reach us by September Expected commercialization by Q1 FY24
**○ Penem Block at Navsari**
Strategic decision to move the penem block to Navsari to reduce the time to market turned out well Civil work complete, Equipment received and Installation complete Commercialization to begin in August 2022 as announced earlier.
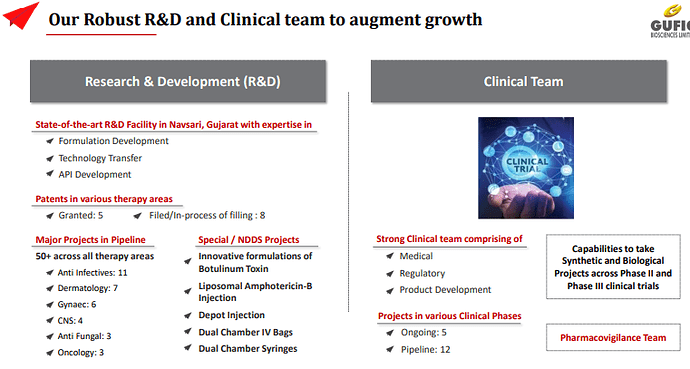
Patents : (Granted: 5 in India)
2015
○ Anidulafungin
○ Tigecycline lyophilized injection
2017
○ Rifabutin
○ Micafungin Lyophilized
2023 **
** ○ Filed/In-process of filling : 8
• Other Updates
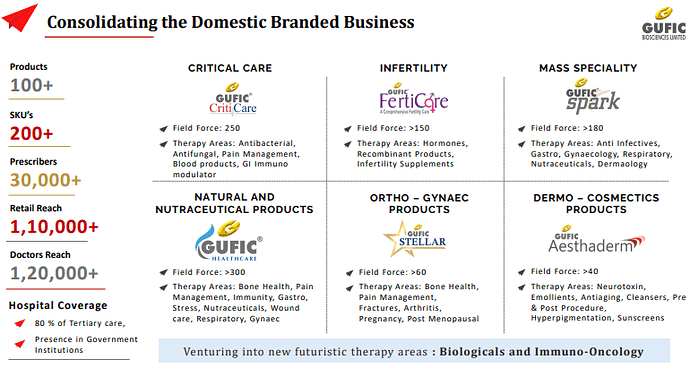
○ Doctor reach: Over 1,20,000
○ Prescribers: 30,000
○ Retail reach: 1,10,000
○ Hospital Coverage: 80-85 % of Tertiary care centres
○ Presence in Government Institutions
Projects in various Clinical Phases
Ongoing: 5
Pipeline: 12
| Product | Reference Drug | Generic Name | Treatment Division | Expected | Launch Date |
|---|---|---|---|---|---|
| D-29 | Dalvance | Dalbavancin Hydrochloride | Novel second-generation lipoglycopeptide Antibiotic | Criticare | Q3 FY22 |
| Stunnox | Botox | Botulinum Toxin Type-A | Anti-Aging injection | Aesthaderm | Q3 FY21 |
| O-26 | Nuzyra | Omadacycline Tosylate | Community-acquired bacterial pneumonia and acute skin and skin structure infections | Criticare | Q1 FY23 |
| IS-6 | Cresemba | Isavuconazole Sulfate | Antifungal to treat invasive aspergillosis and mucomycosis | Criticare | Q4 FY22 |
| Immunocin-α | Zadaxin | Thymosin Alpha-1 | Chronic hepatitis B virus (HBV) infection & undergoing clinical trials for COVID-19 | Criticare | Launched |
| Vancomycin | Market : Germany | Launched 2022 | |||
| Remdesivir | Market : India | Launched 2022 | |||
| Tigecycline | Market : Germany | Launched 2022 | |||
| Anidulafungin | Europe | Yet to Launch | |||
| Micafungin | Europe | Yet to Launch | |||
| Vancomycin | (ex-Germany),South Africa, Canada | Yet to Launch | |||
| Tigecycline | (ex-Germany) | Yet to Launch | |||
| Liposomal Amphotericin-B Injection | Yet to Launch | ||||
| Dual Chamber Syringes | Yet to Launch | ||||
| Dual Chamber IV Bags | Yet to Launch | ||||
| Division | Approx No. of Products to be launched in 12 -18 months |
|---|---|
| Critical care | 6 |
| Infertility | 4 |
| Healthcare | 5 |
| Spark | 5 |
| Stellar | 8 |
| Aesthaderm | 12 |
Herbal : Target
○ Rheumatoid Arthritis
○ Orthopaedic
○ Renal calculi
○ Gynaecological
○ Immunity Boosters and Tonics
○ Gastro intestinal
○ Respiratory
Stridden : A newly created specialty SBU with specific focus on Orthopaedic and Gynaecological products in various segments
○ Pain
○ Infection
○ Pregnancy
○ Lactation
○ Bone and muscle products. Focussed markets are metros
Critical Care Division :
Most of these are Lyophilised injectable - Used in Tertiary Care Hospital (Tertiary care is a higher level of specialized care within a hospital)
○ Anti-Bacterial (Penicillin - based Finished Dosage Forms) - LEADER
○ Anti-Fungal - LEADER
○ Pain
○ Blood products
○ Gastro
○ Neurology Product
○ With the launch of Dual Chamber Bags and normalization of hospitalization we see growth coming back in the next few quarters
Ferticare Division
Most of these are Lyophilised Injectable. - Used in Tertiary Care Hospital
○ Recombinant products
○ Infertility supplements.
○ Menotropin
○ Dydrogesterone: This product is ready for launch. To de-risk they have backward integrated API.
○ Gufic has invested to develop recombinant alternatives to the urinary source of certain hormones which are critical in the treatment of infertility and thereby ensuring we will be independent of geopolitical as well as currency exchange risks and potential pitfalls in the next 15-18 month
Healthcare & Spark Division
Healthcare :Target Ayurvedic doctors and General Practitioners - LEADER in asthama
○ Bone health
○ Pain management
○ Immunity
○ Gastro
○ Wound care
Target General Practitioners and Physicians
○ Anti-Infective
○ Gastro
○ Gynaec
○ Paediatric
○ Respiratory
○ Nutraceutical
○ Cardiac Diabetic Management
○ Anti-Inflammatory - LEADER
○ We are market leaders in anti-inflammatory and herbal medicines
○ We initiated trial of a new product made from an Indian gum by a standardized extraction process for use in the management of asthma
○ Our brand Sallaki continues to be the market leader in Boswellia Serrata
○ The new multivitamin and anti-inflammatory basket should do well in the coming quarters aiding growth in this segment
Stellar Division
Target Orthopaedics and Gynaecology
○ Bone health
○ Fractures
○ Pain
○ Arthritis
○ Pregnancy
○ Post-Menopausal
○ Sallaki Max complimented with launches in the field of pain management and muscle recovery and topical unique oil suspension for better penetration and faster recouperation has led to higher growth in this division
Aestherderm Division
Venture into the potential sub-chronic segment of Aesthetic Dermatology focus on
○ Moisturizing agents
○ Anti-aging
○ Hyperpigmentation
○ Sunscreen
○ Pre/ post procedural products
○ Boutolix i.e. Stunnox
○ Stunnox continues to increase penetration in the market. We are developing fillers to complement and complete this basket
○ Started the training center for new therapies with combination of machines and use of fillers and Botulinum Toxin for face and body contouring
○ Gufic has partnered with experts in the field of vaginal tightening and vaginal rejuvenation and organized training on a national level to promote the use of Botulinum Toxin for these indications
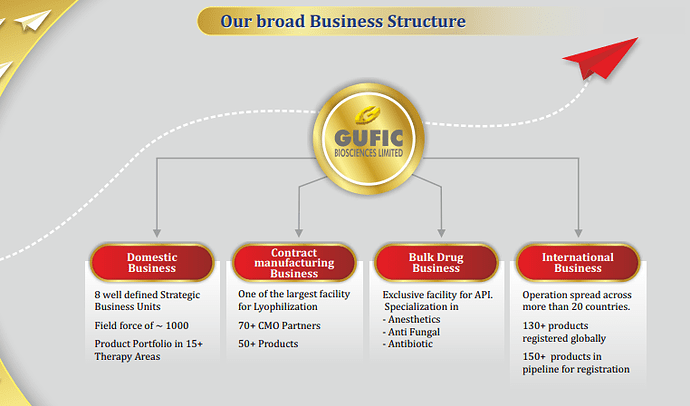
Domestic Business
○ 50% to 55% of our revenue comes from the domestic business
○ 8 well defined Strategic Business Units Field force of ~1,000+ Product Portfolio in 15+ Therapy Areas
International Business
○ In Financial year 2022 we have commenced exports to regulated markets for molecules such as
§ Vancomycin Exported Vancomycin lyophilized injectable to Germany (European Union)
§ Clarithromycin
§ Teicoplanin
§ Tigecycline
○ Have seen growth of ~25% through International Business.
○ Operation spread across more than 20 countries 130+ Products registered globally 150+ products in pipeline for registration
○ Currently 180 Registrations, 13 received and 33 applied in Q1FY23
○ For Europe and LATAM, strategy is in place to register existing developed formulations in countries in which they have presence and enter new countries based on market gaps and opportunities
○ Accreditations
WHO GMP, Philippines BFAD, Nigeria NAFDAC, Cambodia MOH, Kenya PPB, Ethiopia FMHACA, Thailand MOH, Sri Lanka NMRA EU GMP (Hungary), ANVISA Brazil, Russian GMP, Health Canada, Ukraine GMP, Australia TGA, Colombia INVIMA, Uganda NDA, SAHPRA South Africa
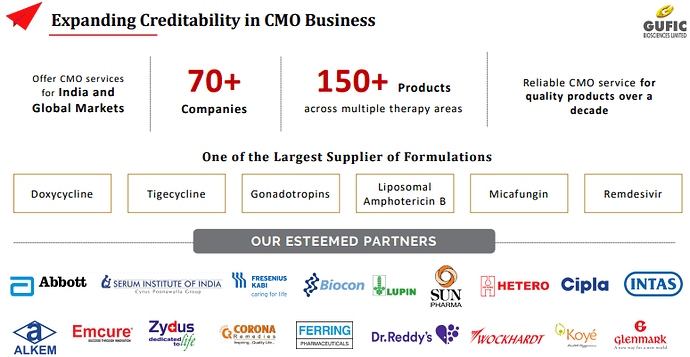
CMO Business
○ CMO market also grow by around below 20%-22%
○ Contract manufacturing division grew by 15% YoY, aided by the merger of Gufic Lifesciences Pvt Ltd & contract manufacturing business of Remdesivir (served ~ 5mn doses)
○ CMO BUSINESS One of the largest facility for Lyophilization 70+ CMO Partners 50+ Products
○ Stunnox brand in February 2021 which was mostly related to the facial aesthetics and we were very keen I think to launch the Gufic Biosciences Limited May 23, 2022
○ Zarbot brand which is our medical I would say related to medical users and specially for neurological uses apart from other uses in pain also
API Business
○ API division grew by 40% aided by increased capacities and substantial investments in product development.
○ We aim to launch 5 new molecules in FY 22.
Centre of Excellence in Mumbai
○ The centre of excellence will treat the skin (not just facial skin) and the body as a holistic organism using most advanced equipment, toxins and fillers for face and body contouring.
○ Moreover, the knowledge repository of the centre will be made open and available to all the members of the medical fraternity across fields, specializations and philosophies to leverage our findings, thus making available the magnificent and marvellous capabilities of botulinum toxin to the society at large
Update on Research & Development and Innovation
○ The API Research Development at Navsari has made noteworthy progress in development of molecules in therapeutic categories such as Antifungal, Anticoagulant, Tetracycline Antibiotics, Progestin, Beta 3 adrenegic agonists, Antidiabetic, Cyclopeptides Hormones. These development projects are all progressing in line with the plan
○ Clinical Trial for D29 will be completed by Q2 FY23 and will be submitted by Q3 FY23 to the DCGI for final approval. This is a novel once a week anti-infective to be launched for the first time in India
○ Biapenem – Approvals are expected soon for vials and for Dual Chamber Bags (first company in India for Dual Chamber Bags). Currently, the market penetration is low due to unviable pricing. Gufic via its pricing and reach will plan to increase the market for Biapenem in India
○ Launched of Dual Chamber Bags for the first time in India.
○ Isavuconazole oral option to compliment the injectable by Q3 FY23. The overall market of this molecule is growing at 100%
Selvax Update : Biotech Company
§ Immuno Oncology
§ It has showed some good clinical success in animal studies (Preclinical stage) and is now preparing for human trials (Phase 1).
§ Thymosin alpha1
○ The Selvax immunotherapy demonstrated promising results (100% long-term cures alongside induction of protective immunity) in the two pancreatic cancer models tested in the pre-clinical stage. These results align with the other different mouse tumour models tested.
○ Moreover, it has consistently outperformed FDA approved checkpoint inhibitors which have become first line therapies for some cancers, including melanoma. These results indicate that the Selvax immunotherapeutic approach could offer a viable alternative to existing therapies for the treatment of pancreatic cancer.
○ Current treatment options for pancreatic cancer include surgery, chemotherapy, radiotherapy, and ablation. These options are rarely effective, and in most cases are used to manage symptoms rather than eradicate disease, highlighting a dire need for new treatments that are effective at combating a cancer that is currently incurable
Next Growth Drivers
○ Gufic aims to achieve 1000 Crores as soon as possible not sure whether in 2024 or 2025 or 2026, but this is their internal target
§ Gufic hopes in the next 3 years, they should definitely grow to a double digit, maybe next 2 to 3 years
○ Gufic hopes to have a double-digit market share in the represented market.
○ Gufic has plans from the Indore factory and from the Navsari factory to actually go and gradually address in the next 3 years a Rs. 25,000 crore market share where they have listed out molecules as per ORG IMS.
○ Earlier Gufic was mainly targeting secondary and tertiary care hospital, now they have started penetrating into primary care hospitals and nursing homes, which is a fragmented but a large and a fast growing
○ Gufic has a very strong field presence in infertility segment and intend to be among the top 3 players in India in the next 3 years
○ With the launch of Dual Chamber Bags and normalization of hospitalization we see growth coming back in the next few quarters
§ Gufic is planning annual sales of Rs. 20 Crs. in less than 2 years for Ferticare Division especially for Dydrogesterone
○ Venturing into new futuristic therapy areas : Biologicals and Immuno-Oncology
○ Zarbot, which is the first Botulinum Toxin in India targeting cerebral palsy migraine and overactive bladder
○ Planning to have 140 molecules to be prepared and launched in the next 2 to 3 years.
○ With Lyophilization as backbone the product extension to support to lyophilization in terms of prefilled syringes, ampoules, suspensions, vials, dual chamber bags to even provision for ophthalmic line also in Phase 2
○ Gufic is also in look out for CDMO opportunities as and when market opportunity for US opens, they are equipped to handle their CMO business or CDMO business.
○ D29 is a novel once a week anti-infective to be launched for the first time in India there is no innovator in India for D29 and also DD29 is backward integrated in terms of API with having limited, no competition at least for the first year in India.
There will be an upgrade to the existing therapy of Vancomycin, Linezolid and Teicoplanin
○ New indications for an existing peptide molecule mainly targeting endometriosis and recurrent implantation failure. The results of studies and initial trials are very, very promising. Both these indications have a large unaddressed market in India
○ To take D29 from Indore factory to the European and the US market where in the product is already approved, the innovator is already there.
Strength
• The capacity for lyophilization is huge. This we’ll be the largest facility manufacturer in the world of lyophilization and it take minimum of 2 years for setting up the plant and getting approvals
• Only company to make Botulinum Toxin
• Complete autonomous manufacturing for Lyophilization process and no manual intervention needed
• High success rate of USFDA approval in Lyophilization injectable space compared to traditional injectable space
• They have the widest basket of products now in the infertility segment and are vertically integrated with own manufacturing API.
• Products widely circulated across 1,500+ hospital chains and leading medical facilities through an extensive network of 1000+ marketing representatives across India
Margins Trajectory
• First year the gross margin may be in the range of around 45%.
• Second year, it can be increased to 50%
• From second year onward the gross margin will be around 55%
Geography
• 2022 entered in two new regulated markets of Brazil and Canada.
Approvals
FY 2020-2021 :
• Rifampicin injections have been approved to be manufactured from GLPL for the Germany market in January, 2021
• 0-26 has been planned to be submitted to the drug regulatory body by the month of July 2021 with an aim of commercialization in the financial year 2022-23.
• IS -6 project has been progressing well and expect an approval in financial year 2021- 22
• Immunocin Alpha trials in Covid are progressing well and with the help of the medical department, the Company has decided to initiate trials in 2 other indications by Q4 2020-2021 which will cement lmmunocin alpha as the biggest brand of Gufic in the shortest span of time.
• Gufic also launched two brands of
• Prefilled syringe molecules in Q3 2020-2021 for cardiac conditions
• Planning at least 3 new molecules in the next 12 months period in the category of biological peptides in the field of gynaecology
• Gufic in Q3 2020-2021 also initiated heavy investments in the development of H 15 candidate for Asthma along with DC 1, DC 2 and DC 3 NODS formulations for Anti-infective use
• Indore Factory inspections will start from, November, December, January itself even before going live with commercialization.
• 3 Contracts out of which two of them already the dossier is in place and going very aggressively, one we might depend on the timelines of when will the Indore commercialization happen and if the client happy time for the ANDA filing.
FY 2022-2023 :
○ Five new product approvals from regulated markets
○ Eight new product approvals from the semi regulated market
○ 13 registrations received
○ 33 new registrations applied
• Applied to DCGI for sepsis indication it is a large market
• Launched Sallaki Max it is a nutraceutical targeting arthritic pain (Stellar Division)
• Completing the clinical trial for D29 by Q3 of FY23, and will be submitting by around the same time to DCGI for final approval
• Interesting work done in biapenem in the 2023 Q1 quarter and expecting approval soon not only in the vial form, but also Dual Chamber Bag and Dual Chamber Bag will be the first company in India to launch biapenem.
• Another thing about biapenem is that currently the market penetration is quite low due to the unviable pricing, but using our proprietary technology, Gufic intends to reduce the pricing and increase the reach and thereby increasing the overall market for biapenem in India
• Current treatment options for pancreatic cancer include surgery, chemotherapy and radiotherapy
• Demonstrated promising results in 2 pancreatic cancer models tested in the preclinical stage by Salvax
2021 Covid Growth - One Time Growth and continuation
• 2021 very strategic developments had happened in Q4 was we Gufic had received permission to manufacture sell and distribute Sodium Sulfate API and the finished formulation which is Isoconazole for injection
• This is an injection targeting patients above 18 years of age for treatment for invasive Aspergillosis and invasive Mucormycosis.
• Beyond this we Gufic has received the DCGI approval for Thymosin Alpha-1
• Immunocin Alpha was a homegrown brand in this molecule, which is used as an add on therapy for treatment of moderate-to-severe COVID-19 patients who require ventilator support
• Thymosin Alpha, which Gufic got approval in COVID we do not foresee much traction in sales in India for COVID
• Immunocin has significantly reduced the risk of death in phase three clinical trials in adult patients with moderate-to-severe COVID-19.
• The domestic market market size of $6 million specifically related to the molecule Isoconazole where there is only one innovator that is of course Pfizer which is there in the market, Gufic foresee that this product is also going to be working against other molecules having a similar therapeutic profile like Posaconazole amphotericin based molecules and also use in combination with Echinocandins so if combined all that market up of course it goes beyond 230 Crores, but management commented that they are in talks about oral permission to receive whereas currently permission is available for injection, management are expecting in third quarter or fourth quarter to receive permission and will be a good basket to take it forward.
Clients
Disc : Studying, not invested