US revenue contributes 44% of consolidated sales grew by almost 50% year on year even it was driven by Zetia exclusivity.Management expect the US base business to grow further to $125 m in Sept q ( from $107 m), and new approval to reflect in Q3 and Q4.Company out licensing deal for its molecules in research pipeline can provide add trigger.Management in it’s call after result had highlighted that it is active discussion for four molecules and expects at least one deal by yr end.
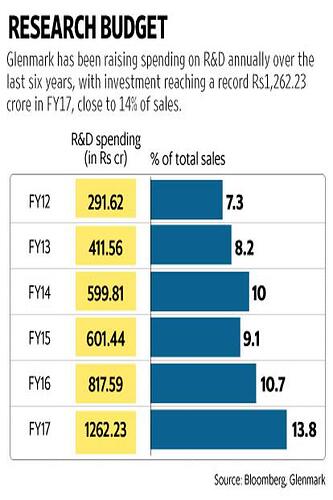
Glenmark has been raising spending on R&D annually despite facing headwinds in both domestic and US markets .
There were recent news about Glenmark reigning in their R&D expenditure to shore up profitability. While Mr. Saldanha says the focus on NME will continue. Hope this is not knee jerk reaction but a adequately weighed strategy.
Copied from below article:
“With the US undergoing structural changes in its market, it has become increasingly important to evaluate R&D spends on commodity generics. Thus, Glenmark will rationalize its R&D spends where it feels that there will be severe competition. Of the total R&D investment, contribution for generics will remain at 4-5% and the rest will be given to specialty and innovation products,” Saldanha said in an email interview.
Glenmark is at 52 weeks low. Good time to start a SIP ?? I understand the issues with US generic pricing pressure due to consolidation among buyers, more USFDA approvals etc but for say 10 year view, I think Indian pharma companies are well positioned.
Even with the recent USFDA observations, stock did not correct at all so looks like it has bottomed out…
Want to find the share of OTC in Glenmark revenue and profits. I see that Vwash is a best seller in its segment and have been noticing TV ads for antidandruff shampoo scalpe+ recently. Also want to understand the economics of OTC vs prescription business. Would be grateful if experienced members could point out some sources.
Thanks
My impression is that OTC should have better profit margins since there is hardly any R&D and you are selling directly to customer (sort of like FMCG)
disc - invested recently
Not activity on the thread for a long time. Seems like the market has given up on Glenmark. One major concern could be the debt.
Looking for opinion about how you would rate Glenn Saldanha in terms of being minority shareholder friendly and sticking to his promises.
GLENMARK_BSE_NSE_22062019.pdf (166.6 KB)
May be this is the reason…
HDFC Securities Report on Q1 FY20 Results
The main reason I believe is that market has lost confidence in Pharma sector and more so in Glenmark. It will take some time to regain that.
They are doing / planning a number of things to reduce debt and restructure their organization for better profitability and efficiency, e.g. spinning off innovation business, divestment of non-core assets, ortho/pain business etc. All these will take time to complete, probably will complete upto next FY.
Further, market is probably waiting to see how their novel drug Ryaltris is received by various markets. New drugs are very expansive to develop and have very less chance of success, but still a decent success of one novel drug can generate enough profit to overcome the failure of multiple new drugs. So, success of Ryaltris is critical for Glenmark. It is yet to release in US after receiving CRL from USFDA in June. It is also releasing the drug in other countries via in-licensing agreements.
It is indeed undervalued but may require a stream of good news to start its upwards journey again.
Such has become the reputation of Glenmark stock that it goes down even on favorable news. ![]()
Moneycontrol: Glenmark Pharma gets Russia’s approval to market allergic rhinitis tablets;
share hits 52-week low.
@sujay85
I have been following this firm quite lately and I think this is very undervalued reason being falling US growth (which is expected to fall more) and leverage.
Management is not able to deleverage the company as mentioned in concalls repeatedly and to add more they are raising 200MM USD more, not sure why.
There is no respite unless some deleveraging comes in here… There non core business needs to be sold off successfully to remove overhang on this…
Any updates from anyone on AGM would be really helpful
Glenmark Pharmaceuticals Inc., USA (Glenmark) has been
granted tentative approval by the United States Food & Drug Administration (U.S. FDA) for
Dimethyl Fumarate Delayed‐Release Capsules, 120 mg and 240 mg, a generic version of
Tecfidera®1 Capsules, 120 mg and 240 mg, of Biogen Inc.
According to IQVIATM sales data for the 12 month period ending August 2019, the Tecfidera®
Capsules, 120 mg and 240 mg market2 achieved annual sales of approximately $3.7 billion
Glenmark’s current portfolio consists of 161 products authorized for distribution in the U.S.
marketplace and 49 ANDA’s pending approval with the U.S. FDA. In addition to these internal
filings, Glenmark continues to identify and explore external development partnerships to
supplement and accelerate the growth of its existing pipeline and portfolio.
Numbers are excellent (compared to expectations)
Beats expections on all fronts
Expected Net Profit 171 crore… reported 255 crores
Expected Revenue 2600 crore… reported 2800 crores
Expected EBITDA 385 crore…reported 450 crores
US Businesses - degrowth expected - reported 5% growth
India Business - single digit growth expected - reported 15-16% growth
I think something is going on here…
Sharing this because bio of Gabriela Gruia is good.
Good Results…2nd quarter in a row… And very interesting updates on Ichnos
http://www.bseindia.com/xml-data/corpfiling/AttachLive/8e8a49cc-06e4-4036-ab06-3460eeb3c74e.pdf
Nirmal Bang initiated coverage on 4th March.
It had fallen to 161.65 levels during the ongoing correction and then resumed to 209.60 level at NSE on the last trading day…
@ayushmit Sir are you tracking this?