Thanks @Meetkatrodiya . Can you please post a link to the doc?
So it looks like Neuland’s expanded capacity could be 120 MTPA + current 40 MTPA = 160 MTPA in bempedoic acid. Blue Jet’s intermediate capacity of 180 MTPA would cater to a bempedoic acid capacity of 140 MTPA (Assuming 1.3 ton of n-1 for 1 ton of bempedoic acid). So total expanded capacity would be 300 MTPA of bempedoic acid (before Blue jet’s debottlenecking effort to increase capacity that is).
Now lets convert this to number of patients who will be catered to. Each patient takes 180mg of bempedoic acid per day - Assuming zero wastage between API → formulation → fixed dosage - this means 1 MT of BA API should cater to…
180 mg per day x 365 days = 65.7 gms/yr per patient
so 1 MT or 1000000 gms works out to 1000000/65.7 = 15220 patients/MT
(Please note this is too simplistic and conservative as there is bound to be wastage due to variety of reasons)
So based on today’s capacity of Neuland at 40 MTPA + Blue Jet’s 180 MTPA of intermediate (140 MTPA of BA probably at Fareva) - 180 MTPA translates to 2.7 million patients.
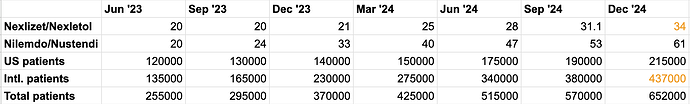
Putting this 2.7 million patients in context - current patients are likely around 650k as of Dec '24 (We don’t have Esperion’s Dec '24 number as earnings not out for quarter - I have made an educated guess based on RPE growth as per Corp presentation in Jan '25)
Since BA’s intermediate would take 9-12 months to end up in retail shelves, its likely that Esperion is planning its supply chain a year ahead based on expected growth. Going by the growth post label expansion, just in current markets of US and Europe, we should see doubling to 1.3 million patients by Dec '25.
Throw in Japan where they should start selling this year and in the near future in Canada and Israel as well. We should also factor in the fact that Esperion hasn’t been able to grow in US market meaningfully so far (Jun '23 revenues in US and Europe were same $20m - today its $61m in Europe and only $34m in US) probably due to issues with insurance coverage, a lack of marketing efforts (having been embroiled in a legal tussle with DSE) - this clearly has potential to grow 100%+ YoY just like Europe, especially since ~30% of the US market is statin intolerant.
Also whatever capacity is being put up is to cater to demand for next 2 years in a scenario of expanding market and expanding acceptance of the drug which should lead to exponential growth and we are probably only at the cusp of the initial stages of the S-curve right now. Along with debottlenecking and a corresponding expansion at Fareva and with Neuland’s expanded capacity, we should have capacity to cater to 6 million patients at peak utilisation, 2-3 years from now. This is not a commodity and there’s only one buyer in the market and all capacity is put up based on order backing and visibility.
Blue Jet might make hay next 3 quarters with debottlenecking until Neuland’s capacity comes online which means 150-160 Cr sort of PAT for next 3 quarters which may drop off a bit after that but even in the worst case scenario the 100 Cr PAT per quarter Blue Jet posted in Dec '24 remains the base case assumption for me and is what we should work with, to be conservative.
The biggest risk is drop in prescription growth which needs to be monitored every quarter. The best case assumption is at least 15-20 million patients on the drug across markets (US, Europe, Japan, Israel, Canada) in 3 years which would be a 25x growth in patients from here.
Disc: Invested