I think market share and physician connect more than price erosion are going to be the driving factors in biosimilars. Given the reliance on the physician to prescribe the brand, the dynamics are more likely to resemble a branded generics market that a pure generics market. So we may not see much more price erosion from here but rather investment in physician engagement, RWE studies, etc. The wild card in all this is interchangeability. So far, no biosimilar has got interchangeability status. Whenever firms figure out the science for that, it could be a big trigger for price erosion.
Positive development for Insulin Glargine
Great News.
I guess getting inter-changeability is also important.
Found this on https://www.goodrx.com/
Basaglar is not a generic equivalent of Lantus, and therefore, the two are not interchangeable. If you are prescribed Lantus and would like to switch to Basaglar, your doctor will need to write you a new prescription. Your pharmacist cannot automatically substitute one for the other without your doctor’s approval
Basaglar is the biosimilar on Lantus.
This is an issue. KMZ had said in one of the interviews that they are trying to convince USFDA about inter interoperability of Samglee. Let’s see if that happens.
I will try to explain the difference between “switching” vs “inter-changeability”
Reference product and let us say there are 2 biosimilars - biosimilar 1, biosimilar 2 - the most common scenario is physicians decide to “switch” from the reference product (expensive) to one of the biosimilars (cheaper). The prescription has to specify which drug - for eg biosimilar2
The above scenario is by far the most common.
EU, Canada and Australia are biosimilar friendly and biosimilars are very successful in these regions. USA is not for a reason - see below…
Interchangeability means that if you prescribe a biosimilar drug with the actual chemical name (not the brand name) - the pharmacist is able to dispense either the reference drug, biosimilar 1 or biosimilar 2. The price might still drop since there are 2 or 3 companies with the same inter-changeable product…
But, to achieve interchangeability status, more clinical trials will have to be done comparing reference product vs the biosimilar - this is very expensive and hence most companies will be happy release their product to the market once their biosimilar product becomes eligible for “switching” from the reference product (cost difference is the main attraction to switching)
In the USA, the prescriber/doctor gets a kickback(rebate or whatever you call it) for prescribing expensive medications (4.5% I read from somewhere). This is the main barrier biosimilars are facing - why would a physician prescribe a cheaper drug since he will be paid less. He gets paid more for using an expensive reference product. I am not making this up, this is true…
Discl - Biocon is 30% of my portfolio and I am very biased
I will recommend this video of Mrs. Shaw from CNBC today (link). Pricing pressure has started showing up on Trastuzumab and Pegfilgrastim to the extent that management is talking about volume growth compensating price de-growth. This remains a key monitorable to establish how much price erosion trends will play out for biosimilars.
From what I gather Lilly’s Insulin Glargine biosimilar also did not get interchangeability status but yet apparently has a 30% market share by volume (Market share data per the earnings call, could someone with access to IMS confirm the same).
Quoting from the following Paper - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6313268/
On interchangeability for Insulin - " The FDA has stated that applications for interchangeability must “include information that demonstrates that the risk in terms of safety or diminished effectiveness of alternating or switching between use of the proposed interchangeable product and the reference product is not greater than the risk of using the reference product without alternating or switching.” The type of studies the FDA will require to prove interchangeability and the safety of switching are currently undefined, with only draft guidance currently in place"
I’m guessing from the above therefore that interchangeability for insulin is still a while away. The commercial implication of the same I am yet to figure out. Any insights from the experts here will be helpful.
Only one biosimilar product is going through clinical trials with the aim to achieve “inter-changeability” status with the reference drug.
But, the point is that vast majority of biosimilar companies will not try to achieve “inter-changeability” status for their biosimilar products- the cost of conducting clinical trial is prohibitive and may not add much in terms of financial gains…
The major reason for using biosimilar is its lower price! If the price difference is not there, why bother changing…
Let us say a company achieves “inter-changeability” status with the originator and the price is almost the same - what will you achieve by using the biosimilar if you are the decision maker - also, most clinicians will stick (or prefer) to the same original brand…
Here is a good overview…
Biocon Ltd (BSE code: 532523, NSE: BIOCON), an innovation-led global biopharmaceutical company, and DKSH, a leading market expansion services provider with a focus on Asia, today announced that Biocon’s subsidiary, Biocon Pharma Limited and DKSH Business Unit Healthcare, have signed an agreement under which DKSH will sell and distribute seven of Biocon Pharma’s generic formulations in Singapore and Thailand. Under the terms of the agreement, DKSH will gain an exclusive license to register and commercialize these seven generic formulations from various therapeutic areas like diabetology, cardiology, oncology and immunology, which will be sold under Biocon’s brand in Singapore and Thailand. DKSH will manage marketing and sales as well as logistics for Biocon Pharma, helping drive sales growth through its capabilities and strengths in the medical and pharmacy channels. This development is in line with Biocon’s strategy for expansion of its generic formulations business through licensing of its drug products in multiple markets. Thailand and Singapore are the two large markets in the South East Asia region
Licensing income expansion from Singapore and Thailand
200 million USD R&D for next year is 1400 crores and that is just above 20% of sales for 2019-20…Highest R&D for a pharma company in terms of % of sales…
Discl - invested and biased
Old article of 2017 but I think still useful
because Biocon comes probably medium sized company in biologics and story unfolding slowly
Some important points from article
CHANGE IN EXPECTATIONS:
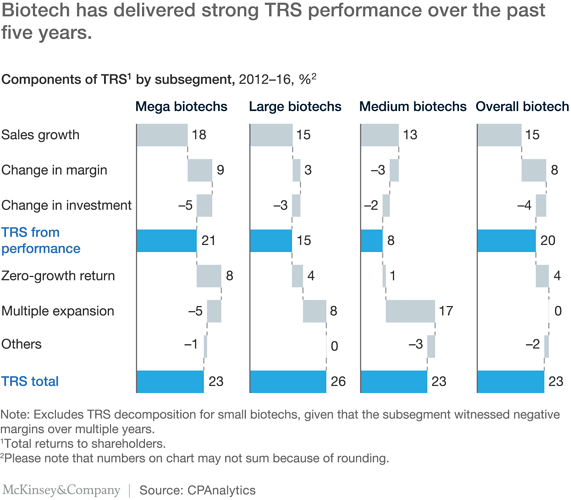
For the next tier, medium biotechs with sales of $100 million to $1 billion, TRS results have mainly been driven by a change in expectations, given that, as a group, they did not outgrow the overall biopharma market (13 percent sales growth) and their margins have remained low or even declined. This change in expectations has likely been driven by investors’ perception that this group could include some of the leaders of the next decade.
MARGIN IMPROVE:
With the improvement in the standard-of-care treatment in primary-care pharma over the past two decades, the highest unmet needs are now concentrated in specialty pharma and orphan diseases—and this is where biotech has focused its efforts. The share of the portfolio focused on orphan-disease drugs has thus increased from 10 to 20 percent in the 2000s to 40 to 50 percent in the past five years. While the development cost on a per-patient basis for these treatments is high, biotech has enjoyed benefits in other parts of its operations. Given that the commercial and patient-engagement model is different for specialty and rare-disease areas, biotech companies have been able to improve their margins significantly by avoiding expensive commercial programs such as large primary-care-physician (PCP) sales forces and direct-to-consumer (DTC) campaigns.
Thanks
The above article is specifically about “innovator biologic companies” - companies that successfully innovate and target new diseases (or provide alternative/novel treatment options) by developing a biologic (usually monoclonal antibody) molecule.
Biocon is not yet such a company - it has not innovated a novel biologic yet but, there are a few undergoing clinical trials. Biocon is a biosimilar company to be precise - their margins are lower than innovator biologic companies… Biosimilar companies copy the original biologic molecule - this process is much harder and more expensive than copying a generic.
But, I am pretty sure they will succeed in developing and be successful in trialing a novel biologic in the not so distant future (their oral insulin and insulin aspart comes to my mind).
Anyway, the share price is appreciating quite nicely against the trend of the broader market. .
Discl - invested and biased
Just add from latest AR.

Biocon got approval from DCGI (the Indian authority) for Itolizumab as a treatment medicine for COVID within India. Any chance FDA appoves it? I guess it will be considered as a NDA because I couldnt find any information on the FDA website. Biocon has been selling this drug since 2013 in India (its available at Rs. 7500/box on Indiamart)
https://www.bseindia.com/xml-data/corpfiling/AttachLive/ee59fd7a-96d0-46d9-b80f-4955c1f4d39d.pdf
Excellent outcome - I did not see this coming. Equillium share price (this company owns Itolizumab) has multiplied in a single day in Nasdaq - Biocon has 10% stake.
More importantly this trial (which reduces mortality/death rate by 30% and reduces hospitalisation days) opens opportunities in other countries and more specifically in the USA…4 injections is the standard treatment and it costs Rs 32,000. The competitor drug is Tocilizumab and a course costs Rs 90,000 and this drug is in short supply…
Discl - Biocon is >30% of my portfolio…very biased
Video explaining Insulia
Biocon Biologics and Voluntis Join Hands for Global Collaboration
on Digital Therapeutics for Insulins
The licensing agreement will make Biocon Biologics one of the first insulins companies to
offer a U.S. Food and Drug Administration-cleared and CE-marked, highly validated digital
therapeutic product, Insulia®, to Type 2 diabetes patients, across several markets in the
world
I think in long-term this will help in better and fast acceptance of Biocon insulin portfolio
Thanks
Most of the type 2 Diabetes may be 90% of them are able to control blood sugar with the help of Diet, exercise and oral drugs… Oral drugs are more comfortable , patient friendly than the injectable insulin…
Only when oral drugs can not control blood sugar (that is when the pancreas stops producing insulin) the type 2 diabetes would need injectable insulin…
In other words , type 2 diabetic patients would take insulin as a last resort…
Given the fact more and more newer oral drugs are being discovered , most type 2 diabetic patients opt for the oral than the injectable insulin.
However, for type 1 diabetes ( the person’s pancreas don’t produce insulin right from birth), no Oral drugs could help…the only way is injectable insulin !