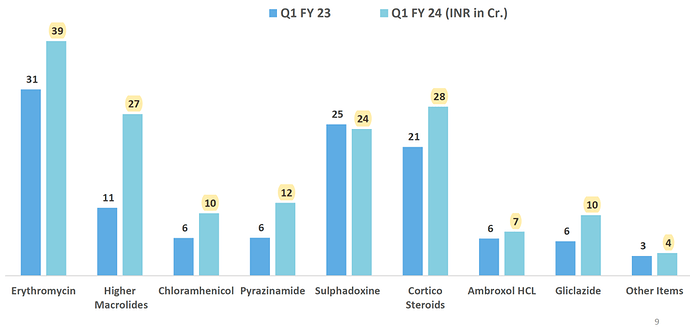
Company came out with a very good quarter, they have increased capacity and got new registrations in EU. I have highlighted key developments from their presentation.
FY24Q1
- Have 8 CEP approvals from Europe (added Gliclazide and Azithromycin this quarter)
- Enhanced capacity from 1500 MTPA to 1800 MTPA
- Undertook commercial validation of vildagliptin for domestic market
- Growth was broad based, domestic market grew by 51% and exports by 26%
- New products under development: Dapagliflozin, Empagliflozin, Canagliflozin
Disclosure: Invested (position size here, no transactions in last-30 days)