What just happened with the Company, poor results. FY 2022 could be worst FY for the Company in last 7-8 years. Extremely poor. Price break just after the Pref Allotment of shares to big funds including Madhu Kela.
Shilpa Medicare historically had two businesses
- API - this is doing well and growing at a decent clip
- CRAMS - this business was sold to the JV partner hence revenue from this line is discontinued.
now they are building three businesses - Formulation - there are some interesting products launched and in the pipeline. however US FDA observation on their Formulation plant means they cant scale in USA till those issues are cleared…
- CRAMS - rebuilding the CRAMS business they have the faciliites trying to get business here… i guess this years revenue is few crores
- Biologicals - this is most interesting as they are manufacturing COVID vaccines for Sputnik and Zydus… Sputnik had issues with manufacture of Sputnik 2 dose hence could not take off in India … now they have approval for single dose Sputnik light… we have to see how is the demand. Zydus is being uses in 15-18 age category in India… so there will be some revenue in the next quarter wrt zydus…
all in all the company is at cross roads and next few years will determine its trajectory.
Disclosure - have tracking position will add at opportune moments…
Would be interesting to understand the impact of this Russia/Ukraine war on Sputnik producers in India. Unless the Govt can work out a way to exchange Ruble/Rupee directly, there could be some short to mid term issues. Russia is being alienated from the banking world and repatriation could be a challenge for some time.
Added to it, Sputnik doesn’t seem to have been picked up well in many countries, which is only going to be exacerbated with the latest situation - as it will be called a “tainted Russian product”.
Sub: Shilpa Medicare Limited (SML) receives NoC from RCGM, Dept. of Biotechnology for its
Biosimilar Aflibercept
Sub: US FDA Inspection of Shilpa Medicare Limited, Unit VII, Analytical Services Division
Hello sir,
Sorry for the naive question I am posing to you. I am unable to figure out how this is beneficial to the existing shareholders. They are shelling out a portion of the company for which every shareholder has contributed. Listing it as an IPO and if they ask the existing shareholders to buy it at discount or bid for fresh stake in the IPO seems to be conflicting with shareholders interest. Isn’t the shareholder going to pay for the stake to which he was also an owner.
In addition to this, if the fresh IPO stock price takes a steep price movement upwards how are the existing shareholders going to benefit from this.
Thanks in advance.
Disc. - Invested
Does look like a pointless exercise. Their entire business is valued at a pe of 64. They are attempting to separate part of the business/earnings into a seperate api business that might give them a higher valuation
There are some companies like Aarti industries that separate the business and issue the shares in the resulting businesses like valiant organics and aarti surfants to existing shareholders thereby increasing shareholder returns and then there are companies like biocon and shilpa that do it for the sake of chasing their own tails or perhaps they are looking to get more external shareholders without getting percentage of promotor equity very low on shareholding pattern
Shilpa Medicare Limited, Unit |, Raichur API facility receives Russian GMP
Shilpa Medicare Limited, Unit VI, Bengaluru facility has been issued
UK MHRA GMP
Shilpa Medicare Limited, Unit III, Advanced Analytical Characterization Laboratory clears
US FDA Remote Record Review.
Shilpa Medicare Limited Completes Human Clinical Studies of
High concentration Biosimilar Adalimumab.
The Drug had global sales of approximately $19 billion in 2021 and is amongst the most valuable drugs on
the market today. The company expects to commercialise the product in the India & RoW markets starting
from the end of the current calender year. SBPL is building a strong biosimilars portfolio around
Autoimmune disorders and Opthalmics — via inhouse development and partnerships with global
companies.
This biosimilar was fully developed at its integrated Dharwad facility. The company intends to ensure
global accessibility to the product via differentiated pricing and formulations/delivery mechanisms.
Cadila Pharmaceuticals Launches Cadalimab (Adalimumab Biosimilar) For India.
https://pharmaintelligence.informa.com/resources/product-content/heres-what-competition-looks-like-in-indias-humira-biosimilars-market
Shilp Concal first time
This is to inform you that the Company has received U.S Food and Drug Administration tentative
approval for its ANDA, Tenofovir Alafenamide Tablets, 25 mg on 15 Sep 2022. The ANDA was filed
as ‘First to File’ submission on NCE -1 date.
https://www.bseindia.com/corporates/anndet_new.aspx?newsid=82c74792-4ada-4785-a020-d376f6c34d1d
Shilpa Medicare Ltd, a Raichur (Karnataka) based pharmaceutical company, dedicated in providing world class Oncology and Non-Oncological products to patients across the geographies, announces yet another innovation in treatment of Colorectal and Metastatic Breast Cancer. The company has introduced Capecitabine 1000 MG dispersible tablet for the first time in the world, with novel technology of faster dispersion within 90 seconds. The Company has launched the product in the Indian market on 16th Dec’22 and is further looking to introduce Capebel 1 gm DT in various international markets through our partners and clients.
They did fund raising on Oct 2021 @ Rs.563/- raised total Rs.297Cr.
Now that value translate to 13.96% of total current market cap. Today Mcap - 2127 Cr.
4 quarters left after that event and which include 2 bad quarter and so much value erosion.
Do you think that at this level it is value buy? still how many quarters it will take to generate potential revenue as per your judgment?
Also request you to share your views here or in An Intelsense Knowledge Series.
Thank you in advance.
10% Interest on FD is for per Annum not per month, what type of calculation it was if they give 10% in a month no one would have reached to this forum for research
Unable to understand the steep rise in Legal & Professional Fees, from 3.63 CR -2017 to 51 CR in 2022 Any idea for the people who are tracking or have position in this company? Also, Payments to Auditor was 11.31 lakhs in 2017 & now 31.63 lakhs it’s been growing above 20% CAGR in last 5 years, Anyone who can throw some light on both of these topics.
it may be related to compliance cost for US FDA .
Notes from May 2023 concall:
- Albumin - Phase-1 started, expected to launch by the end of 2024. No chances of product rejection in phase trials. This product has a 9 billion$ market. It’s an all-over-the-world shortage product.
- Aflibercept - a complex product in the ophthalmic range. Starting phase-3 trials in India. Trying to develop this particular product for the global market.
- Adalimumab - launching in India this quarter, targeting 25 cr. Annual sales
- As per management double-digit growth is definitely possible for the API and the onco, non-onco, polymer, peptide, and CDMO business, each of the divisions will grow this year.
- They have invested 600cr. for Dharwad facility till now.
- Only 75 cr. Capex planned for the coming FY for the product albumin
- All the remediation measures are almost completed. FDA has seen their compliance reports. The date is still unknown for their visit.
- Trying their best to deleverage with all the possible options.
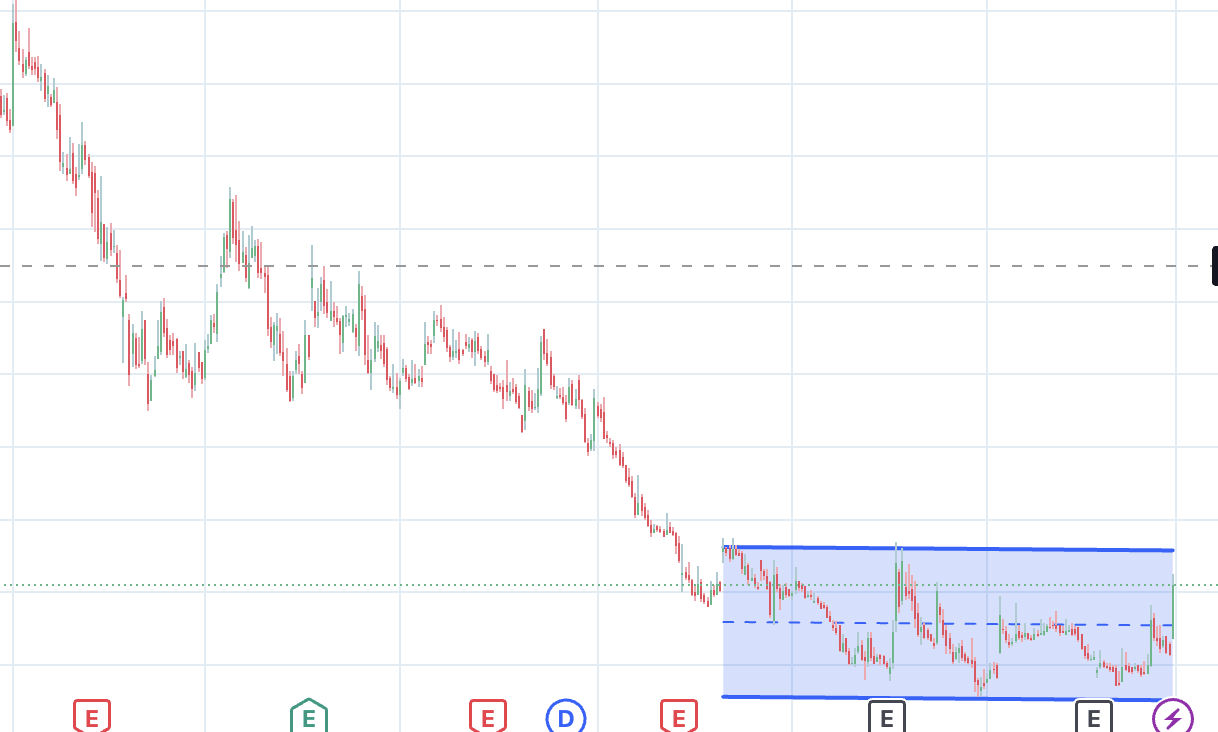
Technically, the stock is trying to form a base around 225. For now, it has stopped making new lows. A breakout from this rectangle might make a case for a turnaround.
The company desperately needs the import alert to be lifted so that margins & cashflows recover, & repairs the balance sheet to an extent. That also gives time & breahting room for other bets/optionalities to play out.
We are delighted to announce that Shilpa Biologicals Private Limited (SBPL), a wholly owned subsidiary of Shilpa Medicare Limited (SML), has received the Marketing Authorization Permission for its Adalimumab 40 mg/0.4mL injection in a prefilled syringe (PFS), a biosimilar of adalimumab higher concentration formulation (100mg/mL) in India. This is used for the treatment of Rheumatoid Arthritis (moderate to severe active RA & severe active and progressive RA) for which the phase 3 clinical trial has been successfully completed in Q3 2022. This formulation will contribute to increased patient comfort based on reduced injection volume.