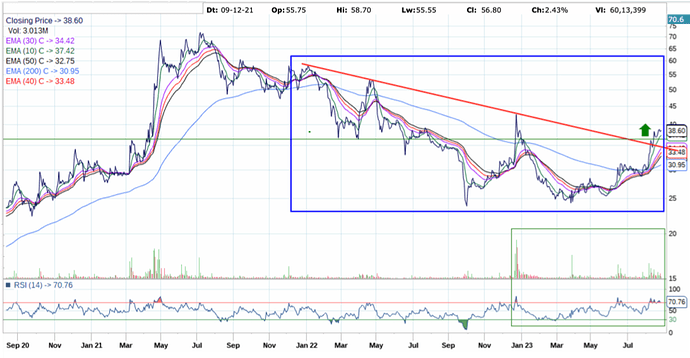
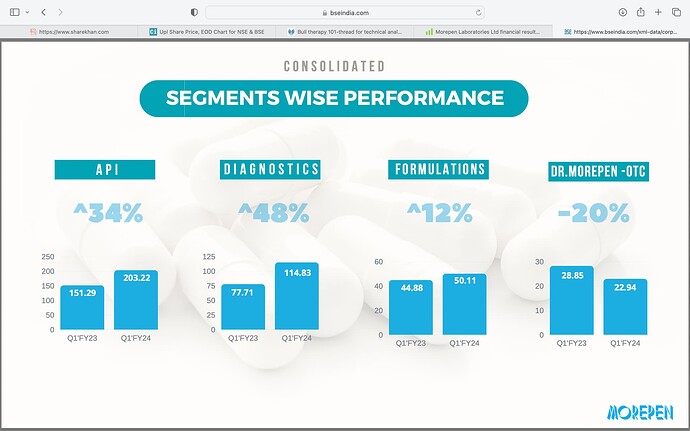
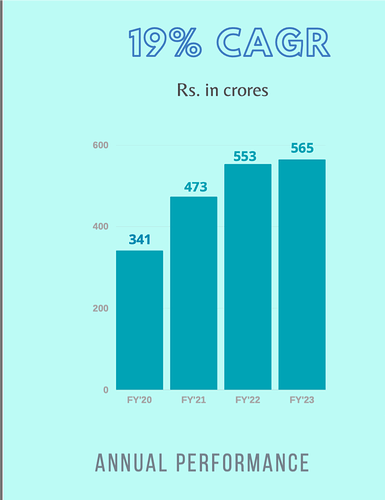
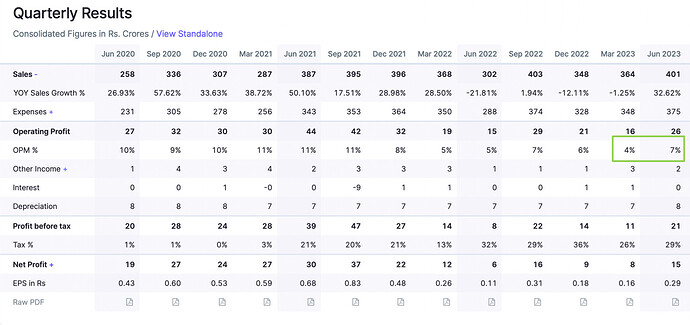
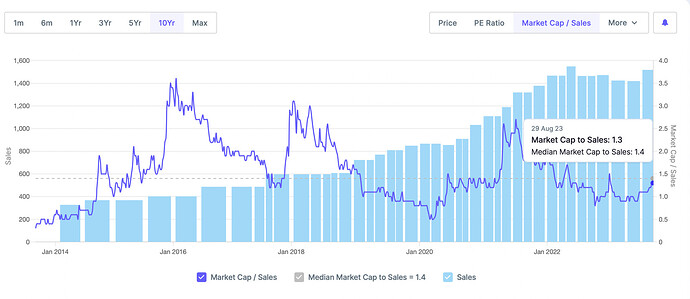
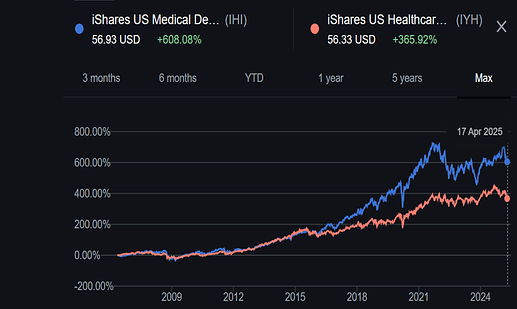
Other then the negative cash flow, everything about this company seems good. But I have issues with the leadership of Mr Sushil Puri, who was also at the helm when corporate governance issues happened. Here is a brief summary.
Here are the key events in Morepen Laboratories’ history, with dates and details on who was leading the company during these times:
1. Debt Crisis and Corporate Debt Restructuring (Early 2000s)
- Date: The financial crisis began in the early 2000s, with the most significant issues arising around 2003-2004.
- Leadership: The company was led by Sushil Suri, who was the Chairman and Managing Director at the time.
- Details: Morepen Laboratories embarked on an aggressive expansion strategy that involved significant borrowing. However, the expected revenue growth did not materialize, leading to cash flow problems and an inability to service the debt. As a result, the company had to undergo a Corporate Debt Restructuring (CDR) process to manage its obligations, which damaged investor confidence.
2. Delayed Debt Repayments (2000s)
- Date: The debt repayment issues continued throughout the mid-2000s, with significant concerns peaking around 2004-2005.
- Leadership: Sushil Suri was still at the helm during this period.
- Details: The company struggled to meet its debt repayment obligations, leading to further financial instability. This prolonged period of financial distress raised concerns about the company’s management and its ability to navigate the crisis.
3. Allegations of Financial Impropriety (Mid-2000s)
- Date: These allegations surfaced in the mid-2000s, particularly around 2005-2006.
- Leadership: Sushil Suri continued to lead Morepen Laboratories during this time.
- Details: There were allegations of financial improprieties, including questionable accounting practices. These allegations led to doubts about the transparency and accuracy of the company’s financial statements. This period was marked by significant challenges in restoring investor trust.
4. SEBI Action for Compliance Issues (2017)
- Date: In 2017, the Securities and Exchange Board of India (SEBI) took action against Morepen Laboratories.
- Leadership: Sushil Suri was still the Chairman and Managing Director.
- Details: SEBI imposed penalties on Morepen Laboratories for delays in the disclosure of financial results and other compliance-related issues. This regulatory action highlighted ongoing concerns about the company’s adherence to regulatory standards and corporate governance.
5. Improvement in Financial Position and Strategic Shifts (2018-Present)
- Date: From around 2018 onwards, Morepen Laboratories has made efforts to improve its financial health and corporate governance.
- Leadership: Sushil Suri continues to lead the company as Chairman and Managing Director.
- Details: The company has focused on expanding its product offerings, particularly in the medical devices and API segments. Morepen has also improved its financial discipline, evidenced by successful fundraising efforts like the recent QIP in 2024. These efforts indicate a positive shift in strategy, though the shadow of past issues remains in investor memory.
and more
https://www.quora.com/What-is-the-MGM-Morepen-scam-in-Delhi
and more
and more
Morepen Laboratories Ltd, a Delhi-based pharmaceutical company, has faced several allegations of fraud, including:
In 2011, the company’s chairman and managing director, Sushil Suri, faced trial for forgery and cheating. The CBI alleged that the company took a bank loan to buy machinery, but then diverted the money, used existing machinery as new, and claimed depreciation benefits from the income tax department.
The Securities and Exchange Board of India (SEBI) barred Morepen from the capital market for a year for making misleading disclosures about global depository receipts (GDRs). The order stated that Morepen pledged the GDR proceeds to loans taken out by subscribers, and that the company violated the Prohibition of Fraudulent and Unfair Trade Practices (PFTUP) norms. The Supreme Court later admitted SEBI’s appeal against the Securities Appellate Tribunal’s (SAT) order, which had issued the one-year ban.
Sushil Suri was involved in all these events. Once a fraud, always a fraud. Also, they diluted significant amount of equity this time which I am not okay with.
Summary
Morepen Laboratories, under the leadership of Sushil Suri, faced significant financial and governance challenges in the early to mid-2000s, which had long-lasting effects on its reputation. Despite these setbacks, the company has shown signs of recovery and strategic growth in recent years, though it is still working to rebuild investor trust.