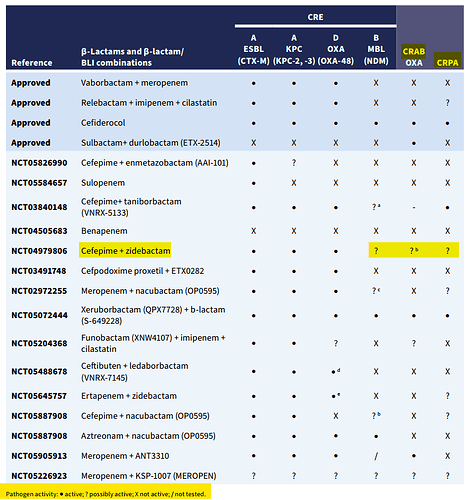
Interesting development for WCK 5222:
https://x.com/EB_Hirsch/status/1805264415844872342
Primer on CLSI and Breakpoints:
Looks like Wockhardt wanted to establish the right breakpoint (64 mg/L) for WCK 5222 for researchers & investigators. This is only the 2nd instance of an investigational antibiotic being assigned CLSI breakpoint. The proposal was approved by the full AST subcommittee:
https://x.com/EB_Hirsch/status/1805315186259374562
CLSI decides the breakpoint for drugs once its approved by FDA. But in WCK 5222’s case, many research papers 1) were using incorrect MIC determination method and 2) didn’t understand the “enhancer” mechanism of WCK 5222.
The reason why some papers consider WCK 5222 MICs of 16 to 64 mg/L as resistant is because they interpreted the data based on cefepime’s susceptibility breakpoint of 8 mg/L. Zidebactam is still under clinical trial and CLSI had not assigned any breakpoint as it was not approved by FDA. Researchers used a fixed concentration of Zidebactam at 4 mg/L which is scientifically wrong as the MIC determination criteria is 1:1.
Also, majority of researchers are driven by MIC90 value and lack ability to assess the drug based on PK/PD data. It is very challenging for experts who have been soaked into only one approach ie. beta lactamase inhibitor to comprehend other approaches.
Good read on how PK/PD impacts breakpoints by EUCAST:
The important point to understand is PK/PD MIC can be higher than MIC90.
Now the “enhancer” mechanism:
Unlike traditional β-lactamase inhibitors, zidebactam not only serves as an inhibitor but also directly exhibits antibacterial activity by effectively binding to PBP2, thereby deactivating this crucial enzyme essential for cell multiplication. Once the zidebactam-mediated PBP2 inactivation has occurred, stage is set for synergy with cefepime that binds to PBP3 and/or PBP1. Hence, we have to look at the combined effects of both cefepime and zidebactam.
The heart & soul of WCK 5222 is this paper by Wockhardt:
Zidebactam Augments Cefepime against Acinetobacter Baumanni.pdf (1.2 MB)
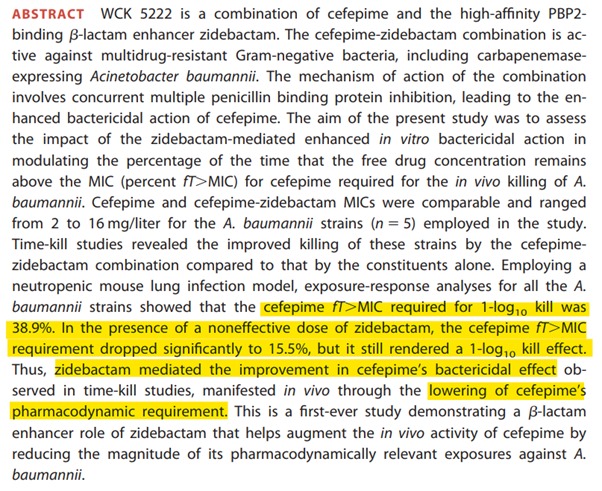
While other drugs have to be present in the blood stream with above MIC concentration for 40-60% of the dosing interval, the combination of Cefepime Zidebactam needs only 15.5% time for 1-log kill and hence can have a higher MIC.
To explain in a picture, Cefepime usually hits 90% target at 60% fT>MIC and hence 8 mg/L but in the presence of Zidebactam, it hits 90% target at 15.5% fT>MIC and hence can have higher MIC of 64 mg/L.
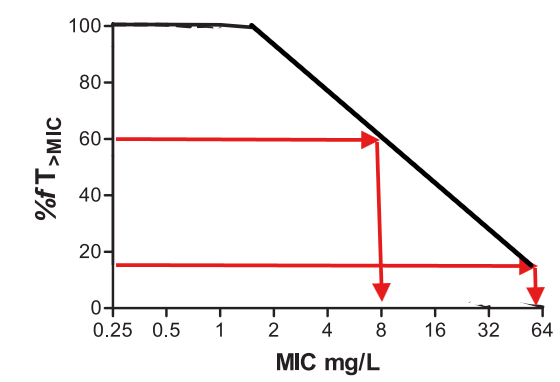
(I have made a simple graph to explain. Real world graph will look a lot different.)
What does it mean in terms of market opportunity?
Going forward, the research & medical community should be using the higher breakpoint for evaluating WCK 5222. We will get to see the real potential of WCK 5222 as it will start being looked at as a drug with a wide spectrum of activity. I think, WCK 5222 is a first-in-class and best-in-class antibiotic drug with the broadest ever therapeutic scope.
Why do I say that?
With an increased breakpoint being assigned, doctors are more likely to use a drug with more steps (or dilution) between susceptibility MIC and breakpoint MIC to reduce the chances of resistance/tolerance being developed or absorption or something else as defined by PK/PD.
Susceptibility MIC vs Breakpoint MIC: Susceptibility MIC of most Enterobacterales & Pseudomonas for WCK 5222 is <8 mg/L going by the data in previous post by Bharani. Since the Breakpoint MIC is now 64 mg/L, the increased steps (3 = 16, 32, 64) between susceptibility MIC and breakpoint MIC means that resistance to WCK 5222 is less likely than those antibiotics with less no of steps. In other words, the bacteria will take a longer time to figure out a way to defeat WCK 5222.
How does WCK 5222 compare with other drugs?
| Enterobacterales (mg/L) | Susceptibility MIC | Breakpoint MIC | No of steps | Remarks |
|---|---|---|---|---|
| Ceftazidime-avibactam | 0.125 - 128 | 8 | 0 - 6 | Inactive against NDM, IMP & VIM Carba strains |
| Meropenem-vaborbactam | 0.125 - 32 | 8 | 0 - 6 | Inactive against NDM & IMP Carba strains |
| Aztreonam-avibactam | 0.125 - 16 | 4 | 0 - 5 | Inactive against PER ESBL strains |
| Cefepime-taniborbactam | 0.125 - 16 | 4 | 0 - 5 | Inactive against MBL producing isolates, NDM & IMP |
| Cefepime-enmetazobactam | 0.125 - 128 | 4 | 0 - 5 | Inactive against MBL & OXA producing isolates, NDM, VIM |
| Cefepime-zidebactam (WCK 5222) | 0.125 - 2 | 64 | 5 - 9 | Active against all strains & classes |
| Pseudomonas (mg/L) | Susceptibility MIC | Breakpoint MIC | No of steps | Remarks |
|---|---|---|---|---|
| Cefiderocol | 0.5 - 16 | 2 | 0 - 2 | Inactive against few strains |
| Ceftazidime-avibactam | 1 - 128 | 8 | 0 - 3 | Inactive against NDM, VIM, IMP and other Carba strains |
| Meropenem-vaborbactam | 0.25 - 64 | 8 | 0 - 5 | Inactive against NDM, VIM, IMP and other Carba strains |
| Aztreonam-avibactam | 2 - 32 | 4 | 0 - 1 | Inactive against PER, KPC and few other ESBL strains |
| Cefepime-zidebactam (WCK 5222) | 1 - 32 | 64 | 1 - 6 | Active against all strains & classes |
| Acinetobacter (mg/L) | Susceptibility MIC | Breakpoint MIC | No of steps | Remarks |
|---|---|---|---|---|
| Cefiderocol | IE | IE | - | Insufficient evidence. Clinical data on efficacy are limited |
| Sulbactam-durlobactam | 0.25 - 4 | 4 | 0 - 4 | Active against all strains & classes |
| Cefepime-zidebactam (WCK 5222) | 32-64 | 64 | 0 - 1 | Active against all strains & classes |
WCK 5222 has the highest number of steps away from breakpoint in case of Enterobacterales and Pseudomonas and is active against ALL strains. In Acinetobacter, only one drug Sulbactam-Durlobactam is superior to WCK 5222. No other drug is even active against Acinetobacter.
So it could be the case that WCK 5222 is the last resort (life saving) drug for Enterobacterales & Pseudomonas and the primary drug for Acinetobacter while Sulbactam-Durlobactam be the last resort. This I think is biggest takeaway for the research & medical community including WHO because WCK 5222 was considered as “possibly active” for Acinetobacter and Pseudomonas. Now it should be considered as “active” similar to Cefiderocol.
WHO 2023:
Actual use of drugs will depend on severity, time available (AST might take few hours), doctors’ choice and many other factors given the “save the best for the last” practice followed when it comes to antibiotics. Also, most countries have protocols regarding usage of antibiotics.
The dosage and concentration used in clinical trials or usage post approval don’t change because of this development. And it doesn’t impact the phase 3 trials going on currently as Breakpoint MIC has nothing to do with trials and is done in-vitro in labs based on bacteria specimens available globally. The FDA had anyway approved 64 mg/L MIC. Dosing will continue to be 2g Cefepime and 1g Zidebactam as decided in the trial design.