As per the ICICIdirect report dated 28 Sept on Alembic pharma, both Torrent and Alembic have a market share of ~10% each for Abilify. Apotex is still too small.
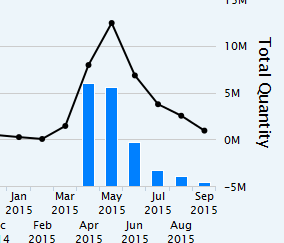
If my reading of Zauba data is correct then export of Ablify(aripiprazole) has dropped drastically from 260 million USD to 30 million USD from last quarter to this quarter. This also may mean that the projection by the analyst is not far off for Ablify.
Hi Gyan,
We should wait for the results to come out. It is also possible that they would have shipped most of the quantities during Q1FY16 including pre launch quantities.
Let’s do some maths here:
Abilify pre genericization had US sales of USD 7.80 billion (source Otsuka and BMS results). Let’s conservatively assume its USD 5 billion. Assuming 50% decline in the prices (much more than 20 - 25% currently), the total market size remains at USD 2.50 billion. Torrent has market share of 10% and should report around USD 225 million sales for FY16.
A sale of 225 million USD p.a. will give us a run rate of 55 million USD per quarter. As they had exported close to 100 million USD last quarter itself and there is a possibility that the sale ramp up would have taken some time in the last quarter, it is highly likely that in q2 they might not have exhausted all the supplies of q1. So, essentially export data of q3 will confirm the real picture to us.
Though export data confirms that they may not have much sale from ablify for this quarter.
Sorry for my lack of knowledge but I have a basic question …How reliable the data from Zauba is?
That is a very good question. Zauba’s data has matched the declared numbers approximately for the last quarter. The accuracy will be known only in the future when we will be able to verify that its data is matching reported numbers for many companies. Though, if someone is interested, they can do the back testing of its data.
The torrent stock looks terribly undervalued to me. If you take FY 16 EPS, the forward PE is only 15-16.
So, even if the PE corrects to 25/30, for high quality pharma company, this could give superb returns. But one has to be patient for these things. It looks like a mis priced bet or am I missing something ?
Re rating after Q2 may be ?
Disc : invested
Novartis may appeal. The last word on this is not yet out !!
PS - invested in Torrent pharma…feel like @sinha124 above. views may be biased
santosh,
While applying PE one has to look at normalised EPS figures. fy 16 would be a one off due to blockbuster performance of abillify and its contribution to profits and sales.
What it does in fy 17 and beyond needs to be considered. However the cash kitty generated due to abilify windfall could provide a lot of options to the management.
With capex being completed, the base business itself should be able to grow 15%. Overall they should deliver a 20%+ growth for next 2-3 years. The windfall from gAbilify can help them retire debt as well as acquire a company to strengthen their portfolio.
They should definitely do an 80+ eps for FY17 and with a conservative PE of 20, we have some upside right now and at least a 25% in next one year.
Hitesh,
I agree, most of the recent research reports, by Emkay, icici etc estimate a Rs 80 fy17 EPS, and have given targets of 1700 + giving a very reasonable valuation of around 18 FY 17 EPS.
FY 16 EPS estimateD is expected to double, to Rs 90 from 44 in fy 15.
What the co does with this cash is also imp, if it acquires something or shares with shareholders.
But one thing is sure that it is a very safe bet with limited downside and there can be upside if Mr M takes notice of the same.
Torrent gets tentative approval from USFDA for g benicar which is an anti hypertensive olmesartan Medoxomil
Here its pertinent to note that the final approval is the important milestone.
Hitesh ji, we have 4 different patent expiry dates (Apr 25,2016 ; Oct 25,2016 , Nov 19,2021 and May 19,2022) for N021286 ( Daichii Sankyo’s Benicar ) . How to make sense out of it ? when will this become a generic drug? ( As per fiercepharma, patent gets expired in 2016 October )
When looked at DMF (2Q2015 file) for olmesartan Medoxomil, very high number of API approvals are there …
2012 sales for Benicar is 2.5 billion $ (source :- fierce pharma ) . How much can we expect this drug contribute to torrent when it gets final approval and launched after patent expiry ?
Hi Hitesh, Firstly, I would like to thank you for sharing your knowledge and wisdom. I also follow you on twitter.
I have question about TP’s abilify sales for 2017 on wards. Since Abilify is a sticky drug - is it possible that Torrent will do similar sales (let’s say they do $200 million in 2016) in next few years?
When abilify was launched then there were only 4 players. Now Apotex has joined as a 5th player. Couple of days back Aurobindo also got approval. Lupin and Sun have tentative approval. Hence prices should crack, but how much that only time will tell.
Rajesh,
as Raj mentioned price erosion should be expected as more and more players get approval. Plus market share also will get reduced over a time.
The main thing here is to see how much the company makes in the limited competition period. And thats where players like torrent have got lucky and were in the right place at the right time. Alembic also benefitted but since it doesnt have its own front end marketing network in US, (which torrent has), it will have to share profits with marketing partner.
Thanks Hitesh and Raj for detailed response. Now, it makes sense why abilify contribution will go down from 2017 on-wards.
Torrent Pharma receives approval for Memantine Hydrochloride (along with Alembic, Jubilant, Unichem, Macleods, Aurobindo and Silarx)…
As mentioned by Ankit in his file on torrent key molecules, Memantine has a $1.4 bn size, though impact on Torrent will not be much as it will be a late entrant with four players already in the market and these 7 getting approval, leading to a competitive market.
Torrent get’s approval for 20mg/40mg gNexium
http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=203636&TABLE1=OB_Rx
