Thanks for pointers. It would be great to analyze the journey of questions lab though these 2 countries have lot of different when it comes to regulatory aspects of diagnostic n stakeholder roles
I like Velumani and I like the business in its present form.
My only worry is that the valuation it is trading at suggest there is a brand moat, which is not true because what matters is the lowest prices you can offer. Players dont have any pricing power. I believe Thyrocare might be one of the last men standing, if there is a price war, given they have one of the lowest costs. But if there is a price war the financials will look very different from the current high EBITDA margins. If this stock was at 20-25x multiple it is still understandable. But at current valuations it looks way to expensive for an industry without any pricing power
Just my 2 cents.
Dear @suru27,
I completely agree with you that there are a lot of differences between India and the US.
The US, despite being a highly regulated and relatively organised market the leading companies control a very small share of the total market.
In India, the threat of the unorganised sector looms. And, in my opinion, the unorganised sector isn’t going away anytime soon.
It employs at the very least one lakh people.
My understanding of financials is strictly ordinary.
Quest and most other diagnostic companies operate at sub 12% net profit margins despite being behemoths.
I don’t know if there’s some sort of price capping.
If Thyrocare can indeed maintain such margins it’s truly wonderful. But I doubt it.
Just my thoughts. I may be totally wrong.
It’s an informative article on the costs and benefits of screening tests by the National Centre for Biotechnology Information
There is a big difference between Thyrocare (capital light model, most testing automated which requires minimal pathologist input in interpretation of reports). Thyrocare chooses what tests they will offer.
vs
Foreign labs in regulated markets such as quest - they run a capital intensive operation (wide variety of tests, many of which requires interpretation by a pathologist or very experienced lab scientist). They cannot choose high margin tests only (this is the biggest problem for such firms in regulated markets) - they have to offer comprehensive diagnostic testing which eats away their profit margins.
It is difficult for foreign firms to set foot in the diagnostic industry in India from scratch! They have to rely on acquiring firms - this is probably the only way in my opinion. The culture of blood testing is also completely different in India - foreign firms will find it difficult.
Discl - Thyrocare 10% of my portfolio and accumulating since IPO.
Dear @stockcollector,
I’d like to state at the outset itself that I’ve no training in this field of science. The source of my little understanding is the literature available on the Internet. Hence, my apologies if I make erroneous remarks.
In the US, CLIA- Clinical Laboratories Improvement Amendments regulate laboratory testing and require laboratories to have approval from the state as well as the CMS- the agency responsible for issuance of laboratory certificates, before accepting samples from humans.
The FDA is responsible for classification of tests on the basis of complexity.
A high complexity laboratory can perform all levels of testing. A moderate complexity lab can perform all tests upto and including moderate level tests. And, tests that have been approved by the FDA for home use are given a waiver.
Also, a laboratory is supposed to perform tests only in the specific specialities or sub specialities it has been granted certification for.
I studied the CMS application form which mentions Hematology as a specialty.
So, if a laboratory chooses to concentrate just on Hematology would it not be allowed to do so?
I didn’t come across any clause that testifies to this effect.
Also, collection of samples doesn’t require CLIA certification. Samples can be collected in one state and can be then sent to a laboratory in another state that is CLIA certified. This would mean that diagnostic companies wouldn’t have to spend a lot of money on maintaining facilities in various states. They can have central facilities in different regions and can receive samples from various parts of the country. I’m unable to understand what may cause their operation to be capital intensive.
Please excuse me if I’ve erred in my interpretation.
Haematology covers only blood counts. So a lab in regulated markets will not allow a lab to offer tests in Haematology only. Thyrocare offers haematology and full range of biochemistry (all amenable to automation).
Dr lal (a good comparison to foreign firms which offers a wide array of tests) offers haematology, biochem, pathology (solid specimens such as biopsies - labour intensive needs pathologist expert reporting), molecular, cytogenetic and other very specialised testing (again very labour and capital intensive, requires either expert lab scientists or laboratory doctor to report)
Dear @stockcollector,
Many thanks for the prompt reply.
I now understand better that Thyrocare operates in segments which are automated, thus reducing expenditure on human resources. But, I haven’t been able to find any regulation that debars operation of clinical laboratories in any one segment.
Your guidance is much appreciated.
To my knowledge no lab in the developed world offers haematology as their one and only service offering. Most of the tests are subsidised by the government in these countries and hence the prices are also fixed/capped.
I am not answering your question - I don’t know the reason either.
My guess is they don’t want to let firms earn excess profits from selected services with high margins, since someone has to offer other lab services with high capital requirements/labour costs. So when they offer the contract to private firms they club lab services with high and low margins together to make it a win win for both parties…
@stockcollector,
The rationale you’ve presented makes sense.
I tried yet again to find rules that compel clinical laboratories in the US to offer services in various specialisations. But, I couldn’t find any relevant literature.
I may be totally wrong but I find it hard to believe that there are indeed such stipulations.
I read some more and found some information.
There are 4 categories of tests in the US:
- Waived Category
- Moderate Complexity
a) Provider Performed Microscopy Category - High complexity category
This categorization authority is vested in the FDA.
In the waived category are the tests most commonly performed.
The waived category labs are very similar to labs in India. Waived Category labs are relatively easy to establish and the standards set aren’t very stringent. Setting standards very high would have led to thousands of labs going out of business.
The moderate and high complexity labs perform tests that are, as the name suggests, complex and require training and experience.
Certification to perform these tests is more difficult to obtain. Inspections are regularly performed.
Since, performance of these tests requires highly skilled human resources and expensive machinery these tests are performed mainly by big chains of diagnostic companies.
And, the reason why these leading American diagnostic companies perform multiple tests in various specialisations could be because every specialisation may not be big enough to fuel growth.
For example- A diagnostics company that’ll restrict itself to say, haematology, or any field where automation is possible, may have very healthy margins but it’s growth will be very limited. Hence, per my thesis, big companies sacrifice margins to attain growth in top line.
They try to play on volume instead of margins.
So, there probably isn’t a regulation to perform a variety of tests but it makes sense from a business point of view.
Yet again, I’m not sure this is the case. I’m just hypothesizing.
I’ll share what I feel about the situation:
In the next 5-7 years, it’s unlikely, but the concerned authorities may decide to regulate the sector . The reason, to my mind, why this may be done is to improve the outsourcing business. Outsourcing of diagnostic testing to India could be a multi billion dollar opportunity. Doing so could help Western companies cut costs. It’s a win win situation. Most Western countries have stringent norms for processing human samples. And, to be eligible for business from them we would have to introduce changes.
And, the regulations are likely to be similar to those in the US.
Local labs, may be given freedom to perform tests, where even if there’s an error the outcome isn’t harmful or life threatening to the patient.
Also, it will ensure local labs don’t lose business as they employ a significant number of people around the country. Very similar to the waived tests in the US which can be performed without an elaborate arrangement.
The complex tests could be for the larger players. So, regulation could be a boon for bigger players. But, then again, the gates will be open for foreign players.
I’m probably naive and ignorant but I’m unable to grasp why foreign diagnostic companies can’t expand in India. More so, companies like Quest have extensive experience of handling the logistics of sample collection and processing. They’ve been able to connect with areas with poor connectivity and get in reach of millions of Americans.
Coming to wellness screening tests I can think of only one company- Thyrocare. It’s the obvious choice. But, would I be willing to try offerings of other leading companies if the price is right? Definitely.
Also, LabCorp and Quest are also focusing increasingly on wellness screening.
So, vanilla diagnostics companies are diversifying into wellness. And, maybe, in the future Thyrocare will diversify into vanilla diagnostics services.
An apt analogy could be Amazon- the online shopping behemoth, expanding in offline retail and Walmart- the offline shopping behemoth expanding in online retail.
My understanding of this industry is basic. If I’ve erred my apologies.
Management has exceeded expectations, the guideline in Pat, earlier said in conf call , looking at 25% growth for fy18.
Disclosure invested since 1year, 8% of holdings
Excellent result and more dividends. 100 crores in mutual fund investments.
100 crore loan to Nueclear (over a period of time) - I am not sure what the intention is?
Also took an equity stake in Equinox labs for 20 crores for approx 30% stake - again not sure what the intention is.
Hi,
I wasn’t able to find this report on their website, where can I access it directly please.
Thanks,
Sanjay
Sunday, Apr 29, 2018
It is reported in their financial statement
Thanks! Found it on the website…
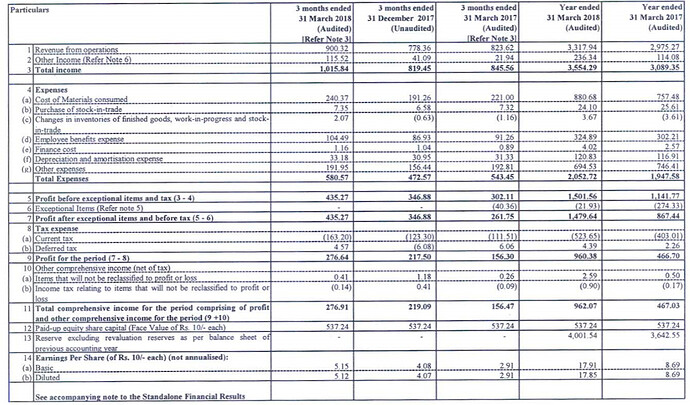
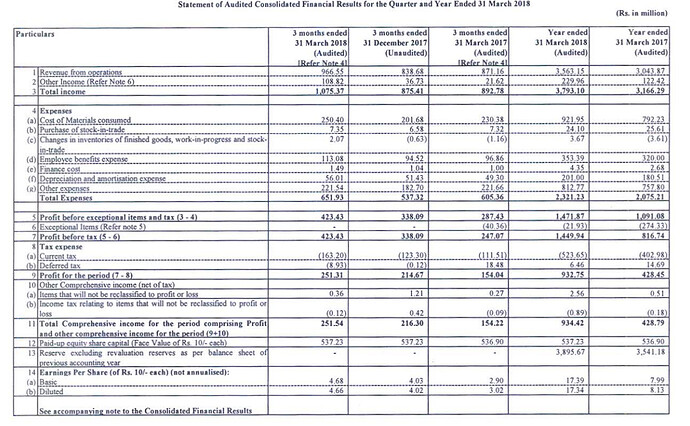
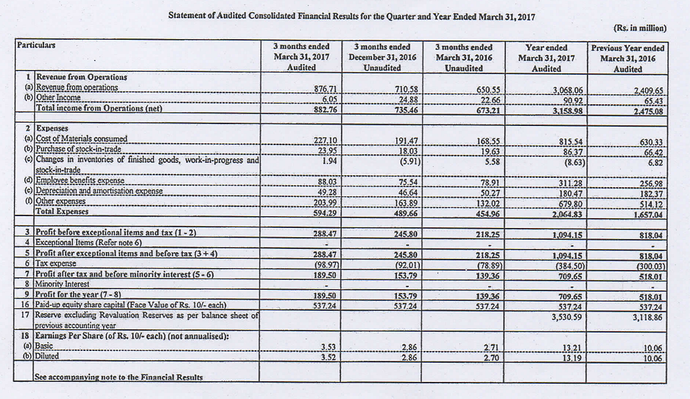
Results look great but the difference between what was reported last year for FY17 and what’s revised this year (due to IND AS, I presume) is quite drastic (Check the Cost of materials consumed and Other expenses which is where large part of the variance is).
Current numbers.
As reported last year EPS for FY17 was Rs.13.19 but in the above report, EPS for FY17 is Rs.8.13. FY18 EPS is Rs.17.34. So depending on what you compare with its a growth of 31% or 113%.
So was Thyrocare trading all of last year at 85 P/E levels and has now come down to 38 P/E? How can we even compare the P/E it was trading in last year with current when there is such a large revision? I am quite confused what exactly in IndAS has made them revise costs so much and if this is the case with other companies as well.
Thanks @phreakv6, if expenses are revised for fy17, does it mean cash flow is also affected? And net cash position last year was incorrect?
4Q results are quite poor. 4Q YoY sales growth was just 8%. In Dec 2017 Velumani had said they will do 25% sales growth in FY18. They did only 16% growth. Competition has started creating structural problems for the company. They are being forced to cut prices to grow. Company is good. But trading at 37x FY18 consolidated EPS it is too expensive. The stock needs to fall to Rs530 for anyone to have any margin of safety
Thank you @RedEPS , this is quite helpful coming from a practitioner. Few questions , if I may -
- I reckon the process works only for blood samples and not any other body fluid. Right?
- What is your mother’s view about sample integrity during the transit - handling, cold storage . Is there any degradation in quality ?
- I would push to ask why there is no viable alternative within Goa . Are we saying that there are some tests that are not possible in Goa … or is it that the logistics /working model works more efficient with Thyro.
Best,
Gary
PS: Invested in Lal … watching Thyro