Notes on Sun Pharma based on latest investor ppt -
No 1 pharma company in India
No 9 in US generics mkt
World ranking in generic pharma- 4th ( behind Mylan, Teva and Novartis. Aurobindo,Lupin and Cipla are ranked -7,8,9 )
43 mfg plants across the world
Present in 100 countries across generic and branded mkts
US business ( 33 pc of sales ) -
9th in US generics mkt, 2nd in dermatology
Present in generics, branded and OTC
US sales for FY 19-20- Rs 10500 cr
India business ( 30 pc of sales ) -
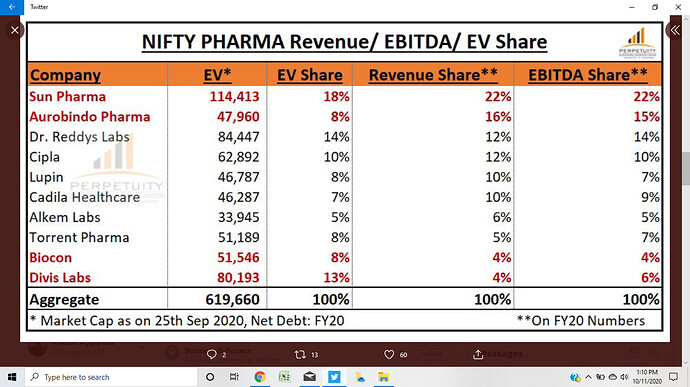
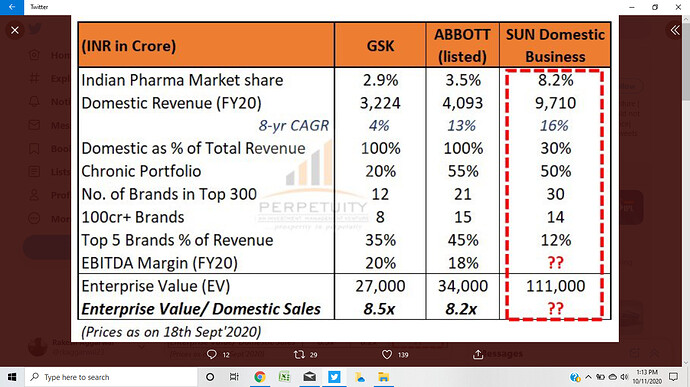
Overall rank- 1 ( mkt share at 8.2 pc ), Cipla and Lupin are ranked 3 and 5
Mkt leader in Chronic segment, strong postn in acute segment
31 brands among top 300 brands
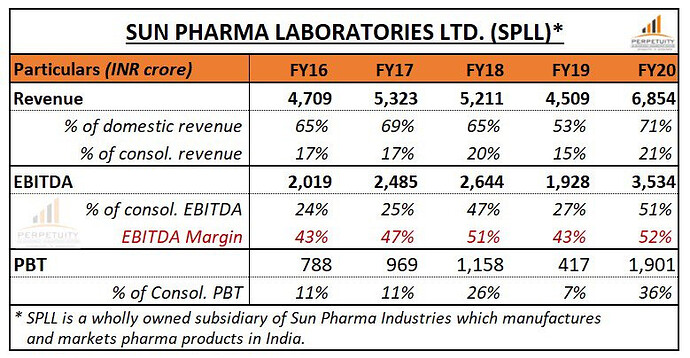
FY 19-20 sales at Rs 9700 cr
Leadership postn among the following Doctor Categories - Psychiatrists, Neurologists, Cardiologists, Orthopaedics, Gastroenterologists, Nephrologists, Diabetologists, Dermatologists,Urologists, ENT and Consulting Physicians
MR field force- 9700
Emerging Mkts ( 17 pc of sales ) -
Present in 80 countries
Focus mkts- Romania, Russia, SA, Brazil, Mexico
Local manufacturing across 07 EM countries
MRs - 2300
Other Developed Mkts ( 14 pc of sales ) -
Include- Aus, NZL, Canada, Japan, Western Europe
Focusing on development of complex generics and other differentiated products
Local mfg at- Canada, Japan, Aus, Israel and Hungary
India and Intl Consumer healthcare -
Brands like- Volini, Revital, Pepfiz, Abzorb etc in India
Also present in - Romania,Russia, SA,Ukraine, Nigeria,Myanmar, Poland, Thailand,Morroco,UAE,Oman - wrt consumer heathcare products
Enjoy strong brand equity in 4 countries ( India, Myanmar, Nigeria and Romania )
Dedicated field force in each Mkt
APIs ( 6 pc of sales ) -
Make 300 APIs
Provide backward integration
Aprox 20 APIs scaled up annually
14 API manufacturing facilities
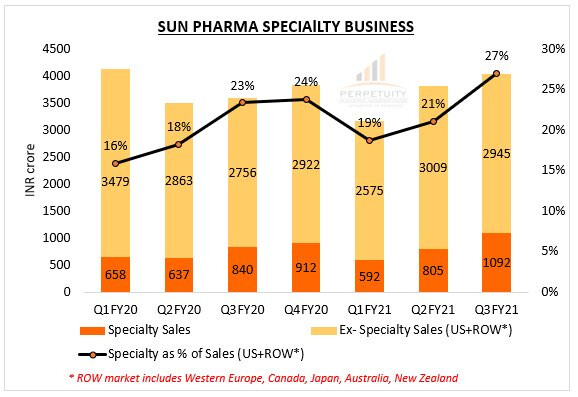
SPECIALTY PORTFOLIO -
ILumya - for plaque psoriasis , launced in US in 2018, Europe and Aus - Dec 18, Outlicensed for Greater China, Japan approval in Jun 2020
Cequa - for dry eye disease, US launch in Oct 19, Outlicensed for greater China Mkt
Absorica- for severe recalcitrant nodular acne, currently being sold in US
Levulan - for actinic keratosis, currently being sold in US
Odomzo - for LABCC, being sold in US,Germany, France, Switzerland, Denmark, Aus, Israel
Yonsa - used in prostate cancer, sold in US
Bromsite - used after catract surgery to relieve pain and inflammation, sold in US
Xelpros - used in ocular hypertension, sold in US
Infugem - a chemotherapy product, being sold in us and Europe
Key deals -
Acquired POLA Pharma in Japan in 2018 , access to Japanese derma mkt
Acquired Global rights for Cequa and Odomzo in 2016
Acquired Biosintez in Russia for local mfg footprint in 2016
Acquired 14 brand from Novartis in Japan in 2016
Acquired Insite Vision in US in 2015, strengthens opthal portfolio
2015 - merged Ranbaxy with itself
2012 - acquired Dusa pharma in US in 2012, got sterile injectable mfg capability in US
2010 - Acquired Taro Pharma ( Israel ) , access to US derma mkt
1997- Acquired Caraco entry in US mkt
Disc: invested drom Rs 480 levels


 .The journey over the years and the organisation as a whole.
.The journey over the years and the organisation as a whole.