In many cases these subsidiary accounts would be unaudited. Been evaluating this - very likely USFDA issue will settle down (many companies face that) but diversification into consumer products (completely unrelated area) and having numerous subsidiaries raise red flags.
From the article
While RDIF has tied up with Gland Pharma (252 m), Hetero Biopharma (100 million), Virchow Biotech (200 m), Panacea Biotech (100 m) and Stelis Biopharma (200 m), which translates into 426 million people equivalent doses, Dr Reddy’s has a tie-up with Shilpa Medicare for an undisclosed number of doses.
It can be recalled that couple of weeks back they were all over the press about their capacity to produce 200 million doses
Announcement on 3-year agreement with DRL for production of Sputnik V. Target production is 50 million doses for first 12 months. They are also exploring the manufacture of Sputnik Light (single-dose vaccine) in the near future.
Shilpa Medicare has received an in-principle approval from Defence Research & Development Organisation (DRDO) to manufacture and sale of 2-Deoxy-D-Glucose (2DG). 2DG has been given emergency approval by the Drug Controller General of India (DCGI) for COVID-19 patients in the country. Shilpa Medicare is only the second company in India to have entered into similar arrangement with DRDO.
The Board at its meeting held today, approved the transfer of API Business of the Company consisting of Unit-1 and Unit-2 situated at Raichur, Karnataka by way of slump sale…
…https://www.bseindia.com/xml-data/corpfiling/AttachLive/64e73262-1920-4ec4-b9ff-760945e7edde.pdf
Company has two platforms line which can be utilised for making COVID vaccines. The first one being for mammalian adenoviruses and the second one is MRNA/DNA. The first one has been tied up with Dr Reddy Ltd for SPUTNIK vaccine which will hit the market sometimes in October.
The Contract is for 100 Million doses per year for 3 year i.e 300 Million doses. The Company is in talks with MRNA/DNA vaccine makers(Pfizer, Zydus,Moderna…) also for tie up for its second platform and the deal may happen within 1 month.
Apart from this the Company has launched Oral Paracetamol in India.The Company is the first one to launch in India.
Recently veteran Pharma Investor Madhu Kela also has purchased around 1000000 Shares of Shipa.
SII, the world’s largest vaccine manufacturer by volume, has already received cell and vector samples from Russia’s Gamaleya Centre, RDIF said.
Disclosure : Invested recently and forms 1% of my portfolio
Shilpa Medicare Limited (SHILPAMED) today announces receipt of confirmation of WHO - GENEVA approval for API, Tenofovir Disoproxil Fumarate, a medication used to treat chronic hepatitis B and to prevent and treat HIV I AIDS, under pre-qualification
program.
Shilpa Medicare Limited is pleased to announce that, on successful confirmation of
Prequalification of API, Praziquental by WHO-Geneva. Praziquantel is used to treat
schistosoma (infection with a type of worm that lives in the bloodstream) and liver
fluke (infection with a type of worm that lives in or near the liver). Praziquantel is in
a class of medications called anthelmintics. It works by killing the worms.
I guess it is animal API
I have a query -
-
What will be the effect on shareholders of Shilpa Medicare as management is transferring the API business of the company by way of slump sale as “going concern” to Shilpa Lifesciences Private Limited ?
-
Slump Sale shall unlock the value for API business, but it wont give any benefit to shareholders of Shilpa Med, as shareholders wont get anything here?
-
What if the management had de-merged the API business, thereby creating more value for its current shareholders. Current shareholders could have got shares of new company (de-merged entity), So is the decision of slump sale detrimental to the interest of current shareholders of Shilpa Medicare ??
-
What will be the effect on Share Price on Shilpa after slump sale ?
need to know opinion of learned members here.
@Rafi_Syed
Listen to the FY22Q1Concall Time Stamp : 23:20 , there are challenges in setting up the cell lines , Arun spoke about contamination etc…
Shilpa medicare has also tied up with DRL for Sputnik. Will they also face similar challenges for seting up of cell lines like Strides Pharma is facing.
Investor Presentation
Anyone holding it and can throw some light on it ??
Disclosure of management are very poor so we will never know if they face challenges. USFDA compliance is very poor and casual approach. Pipeline is robust, planning is excellent but excution is very poor.
I just wanted to understand whether Shilpa and Strides working on similar manufacturing capabilities for SPUTNIK???
I understand that these company have long gestation period and I am prepared to stay invested in this company for long term 5 to 6 years.
As per investor presentation the Company has strong pipe line.
| Regulatory Submissions | Filings | Approved | Pending |
|---|---|---|---|
| US ANDA: SML | 24 | 13 | 11 |
| US NDA: SML | 3 | 1 | 2 |
| US ANDA: Customers | 18 | 12 | 6 |
| TOTAL (In US) | 45 | 26 | 19 |
| EU Filing | 24 | 17 | 7 |
| Row Filling | 228 | 65 | 163 |
| TOTAL (In EU & ROW) | 252 | 82 | 170 |
| GRAND TOTAL | 297 | 108 | 189 |
Just wanted to understand that Company has also given status for Patents with regard to API formulations etc… what are these patents related to and secondly the Company as filed 3 NDA also, so are these NDA related to filing of drug as innovator??
| Patents Filings | Granted | Pending | |
|---|---|---|---|
| API | 205 | 40 | 165 |
| Formulation * | 179 | 26 | 153 |
| Films Topical & Transdermal | 59 | 6 | 53 |
| Biologicals | 12 | 4 | 8 |
| Others | 22 | 5 | 17 |
| TOTAL | 477 | 81 | 396 |
Veteran Pharma Investor Madhu Kela has entered the stock
- Shilpa Medicare Limited enters Phase I Clinical Trial for its New Biological Entity, recombinant Human Albumin
Shilpa Medicare Limited, Unit III R&D facility clears US FDA Remote Record Review with no observations.
Material capex over the past few years has brought down the asset turnovers, implying underutilized capacity & potential for good sales growth over time.
However, there are certain warning flags here. Poor previous 5 years cash flow conversion & increase in debt, long history of repeated equity infusions, ‘trying to do too much’ mindset by signaling numerous areas for growth, minor investments in private cos under ‘strategic’ investments, low dividend payouts etc.
A turnoff is its low depreciation rate historically ~5% vs US focued peers.
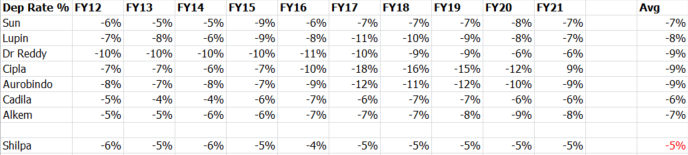
About ~20% of its total block is intangible FY21(80% tangible, 20% intangible). Within intangible, interestingly, bulk is ‘product under development’. Lenient on capitalizing a decent chunk of R&D vs outright expensing it?
Not an accounting expert, someone can further look and give a better perspective, for larger US focused peers, bulk of their intangible assets are primarily brands or trademarks & designs or patents/marketing rights/licenses. Stuff like this.
Shilpa’s Pemetrexed Injection 1000 mg/100 ml, 500 mg/50 ml & 100 mg/10 ml, Ready to
Use formulation is approved by European Authorities
According to IQVIA MAT Q2’2021 (June 2021) data, the EU market for Pemetrexed Injection is
approximately EURO 519 Million.


