Some background Around Orchid Pharma and my notes
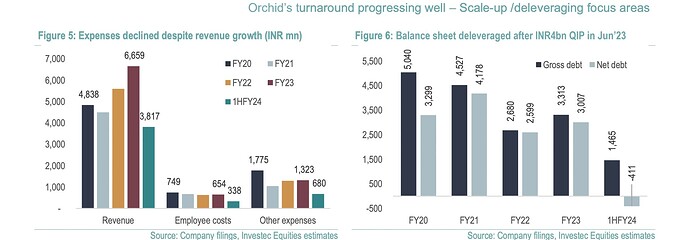
- Orchids Turnaround
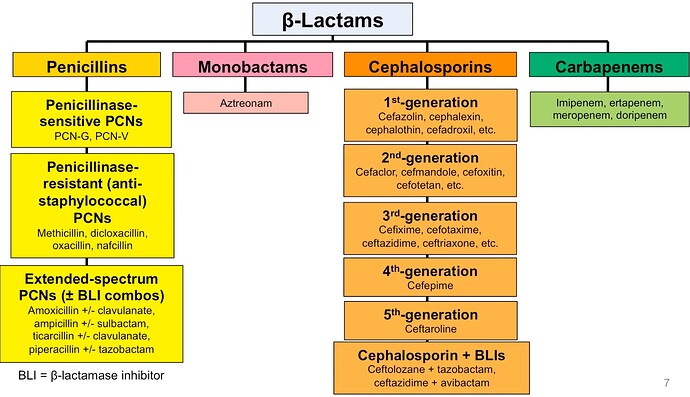
Cephalosporins (Orchid’s focus) – Largest sub-class within beta lactam
-
There are six generations of cephalosporins while the third generation onwards have a strong safety profile and wide coverage of gram-negative bacteria. Orchid has the widest portfolio of more than 30 products across oral and sterile cephalosporins. It has developed products across four generations of cephalosporins and is now developing the fifth- and sixth-generation products.
-
Cephalosporins account for 50%+ share of beta Lactam class
-
Cephalosporins market – API market valued at ~$2bn
-
Cephalosporins have become the preferred class due to their efficacy against a wide range of bacteria and are also well-tolerated by the patients (fewer allergies).
-
Orchid owns the only US FDA approved sterile Ceph facility in India.
-
2017 Orchid went “under” and Dhanuka group acquired in 2020 and Dhanuka already had a Ceph API
business housed under Dhanuka Laboratories Limited (DLL). -
Company has said it will produce 7ACA which is critical raw material in cephalosporin production through PLI scheme.
-
Top three products contribute to roughly three-fourths of Orchid’s sales. Cefixime, Cefuroxime Axetil and Cefdinir.
-
Orchid will hold 20% in Allecra.
-
historically for Orchid first half has been 40% and second half has been 60%.
7-ACA Project
- 7-Aminocephalosporanic acid (7-ACA) is a key intermediate in the production of semisynthetic cephalosporin antibiotics.
- In July 2023, Orchid Pharma announced that its subsidiary, Orchid Bio Pharma, has received approval to manufacture 7-ACA product with a committed capacity of 1,000 tonne per annum. The 7-ACA project is under the production linked incentive (PLI) scheme.
- Registration of the land acquired for 7-ACA project is under progress.
- The strain has been received from their technology partner for pilot plant development.
- 25% captive use + 55% downstream intermediates + 20% external
Cefiderocol
- It’s listed on the WHO Model List of Essential Medicines.
- Orchid Pharma has gained a sublicense agreement to manufacture cefiderocol as part of a global effort to expand antibiotic access worldwide.
- Expertise in cephalosporins will be advantageous in manufacturing affordable and quality-assured cefiderocol,
- Expect to initiate commercial production by 2HFY26.
NCE Enmetazobactam
- Exblifeb incorporates Enmetazobactam : The first completely invented-in-India Beta Lactamase inhibitor. Exblifep | European Medicines Agency
- Enmetazobactam was invented in India by Orchid and then out-licensed to Allecra Therapeutis for further development.
- Recently they have received favourable recommendation for approval from EUCHMP
and expect USFDA approval soon. - The company is in talks with DCGI for their nod to waive clinical trials in India. Dialogues are in progress, if waived, can launch immediately or else will get deferred by 6 months to 12 months.
- The company will receive sales royalty of 6-8% sales worldwide
- Orchid holds royalty rights on global sales and marketing rights in India.
- Pfizer had a similar product ‘Zosyn’ - Enmetazobactam has higher efficacy of 79% vs 59% - So math works out for Orchid too.
Capex in Sterile
Orchid’s existing sterile facility is operating at 100% utilisation and thus the company has expanded the sterile capacity in 2QFY24, which again will be fully utilised by 4QFY24.
Expansion in Oral Capacity
Company’s oral capacity is also operating at high utilization of 75% and so it is expanding oral capacity as well (which will commercialize in 1HFY25).
Jammu’s PLI
Jammu to strengthen NPV and financial viability of the 7-ACA project. Apart from the PLI incentives available under Production Linked Incentive (PLI) Scheme for Promotion of Domestic Manufacturing of critical Key Starting Materials (KSMs)/ Drug Intermediates and Active Pharmaceutical Ingredients (APIs) in the Country, the Jammu location provides additional meaningful benefits to Orchid
- Power is a key component of the total cost of manufacturing 7-ACA, and Jammu has
the lowest power cost in the country, - under the New Central Sector Scheme for Industrial Development of Union Territory of Jammu & Kashmir, ORCHP will be eligible for GST refund at 18%
- Cumulative PLI incentive of Rs. 6bn
Q3FY24
- Revenue : 10% QoQ 38% YoY
- Low Cost inventory : GM expanded
- EBITDA margin @16%
- Operating Leverage benefit should kick in but this Qtr it (Sterile ) caused Opex
Land acquisition in Jammu
Antibiotics
Would highly recommend Bharani’s Sir VP post.
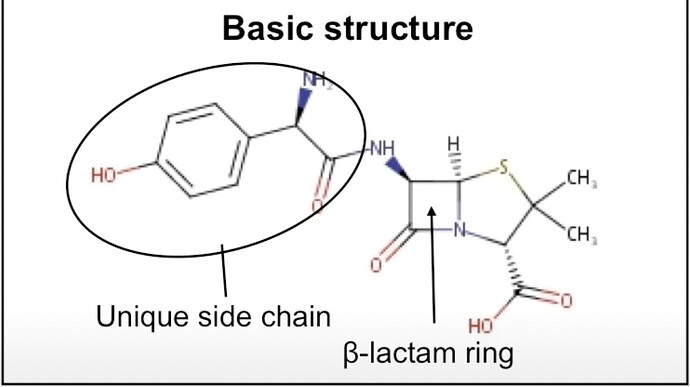
Beta Lactum
Does serendipity help pharmaceutical companies rake in megabucks?