when is the demerger happening? which entity would be more attractive after demerger
ubilant Pharma partially redeems 4.875% Senior Notes Due 2021 aggregating USD 100mn
Someone please explain what is the of note here ? Any links to read more and understand ?
The Ex date for demerger is Feb 04.
As part of the scheme of arrangement the company will issue 1 share of Jubilant Ingrevia Limited (LSI or Life Science ingredients Business) for every share held in Jubilant Life(now name changed to Jubilant Pharmova Limited).
@Krishna1 , where do I check if i have been issued the share of Jubilant Ingrevia. I guess it is not listed yet?
@Krishna1 check your Demat holding, those have been credited long back.
Jubilant Ingrevia shares were listed on March 19,2021.
If you were holding the shares prior to the ex-date, you should have received the shares as @Shivanand_Pandey ji has stated.
A few days ago the company also held an investor call:
Analyst Day Presentation:
Thank you both, I see the shares in Demat list. Was wondering which one has better prospect - Ingrevia or Pharmova , I know both are in different segment, but if i want hold just one, which one should that be?
I think Ingrevia is undervalued as per pees of same sector. Trading at roughly 4100 Crs market cap with expected EPS of 18 gives a PE calculation of 15. I have added to my position received from demerger.
Also, created a separate topic for Ingrevia discussion here
#####Manufacturing contracts from Nonavax and Eli Lilly
23 March 2021
Jubilant HollisterStier Announces COVID-19 Vaccine Candidate Manufacturing Agreement with Novavax
Jubilant HollisterStier LLC, a wholly owned subsidiary of Jubilant Pharma Limited, announced that it has entered into a non-exclusive manufacturing agreement with Novavax, a biotechnology company developing next-generation vaccines for serious infectious diseases, to provide fill-finish manufacturing services for the production of COVID-19 vaccine candidate NVX-CoV2373. Under the terms of the agreement, Jubilant HollisterStier’s Spokane, Washington facility has begun production activities of NVX-CoV2373 final drug product intended for commercial distribution in the United States.
09 March 2021
Jubilant HollisterStier Signs Manufacturing Agreement with Eli Lilly for COVID-19 Therapeutic Bamlanivimab
Jubilant Life Sciences Ltd (“Jubilant”), an integrated global Pharmaceuticals and Life Sciences Company, recently announced that its wholly-owned subsidiary, Jubilant Pharma Limited, through one of its subsidiaries, Jubilant HollisterStier LLC (“Company”), has signed new contract with Eli Lilly for contract manufacturing of a treatment for COVID-19. The drug will be manufactured at the Spokane, Washington, USA facility of the Company.
(CNN)The US government in coordination with Eli Lilly said it will no longer distribute the Covid-19 monoclonal antibody therapy bamlanivimab for use on its own. The halt is due to the “sustained increase” in coronavirus variants in the United States.
…
Bamlanivimab can still be used with etesevimab, another monoclonal antibody treatment developed by Eli Lilly. In combination, the two Eli Lilly treatments seem to work against coronavirus variants.
…
Lilly is currently working with Amgen to scale up the manufacturing of etesevimab
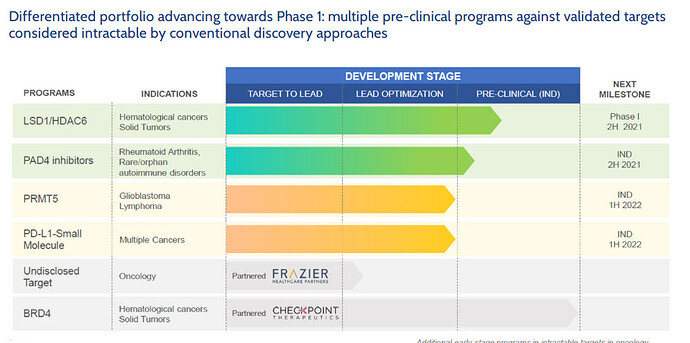
Some activity on the novel drug discovery business…JUBILANT HERAPEUTICS:
10 April 2021
Jubilant Therapeutics Presents Preclinical Data on its Brain Penetrant PRMT5 Inhibitor and Small Molecule PD-L1 Inhibitor at the American Association for Cancer Research (AACR) Annual Meeting 2021
449pressfile_JubilantTx_AACR2021 (1).pdf (347.4 KB)
08 March 2021
Jubilant Therapeutics Announces Appointment of Luca Rastelli, Ph.D. as Chief Scientific Officer
445pressfile_PR-JubilantTx-8March2021.pdf (135.1 KB)
25 February 2021
Jubilant Therapeutics Announces Research Collaboration with Boston Children’s Hospital, Harvard Medical School, to Evaluate PAD4 Inhibitors in Autoimmune/Inflammation Disease Models
444pressfile_PR-JubilantTxBoston-Ch (2).pdf (222.2 KB)
Some very interesting comment by Mr. Hari Bhartia during latest concall…pls. listen from 38 min…Co. is good at early stage science and they’ll look to out licence fro commercialisation to big pharma at the end of phase one or early phase two of clinical trials…
There going tobe success and failure but have portfolio of molecules…
Also,out licencing will limit the cashburn…
Researchbytes.com!
Progress of different molecules…
Discl. Invested since long.
Jubilant Pharma Limited, a subsidiary of Jubilant Pharmova Limited, announces successful completion of safety and pharmacokinetic/absorption studies in animals and healthy human volunteers in India using a novel oral formulation of remdesivir against the commercially available injectable formulation of remdesivir.
This is also positive for Jubilant Ingrevia as they supply key ingredients for Remdesivir.
Thanks!
Any idea when results will be out?
Finally long legal battle won by the company…
JUBILANT.pdf (2.2 MB)
Thanks for sharing this.
Can you throw some light on the likely impact of this favorable judgement on Company’s revenues / profits etc.
That would be really helpful.
Well, in many concalls management didn’t share exact nos. about RUBY-FILL siting secrecy in limited competition arena…its a duopoly market and Bracco fought tooth & nail to protect there turf…last yr. jubilant balance sheet shows a few million USD spent as a legal fees which shows there is booty on the table. Cardiac Positron Emission Tomography (PET) imaging is the gold standard diagnostic test in nuclear cardiology…The average PET scan cost in the United States is $5,750… Exact RUBY-FILL cost not able to find though…
Came across this2013 article filed by POSITRON to the SEC…
https://www.sec.gov/Archives/edgar/data/844985/000114420413004738/v333310_ex99-1.htm
One para from the above article which emphasizes duopoly…
- RADIOPHARMACEUTICALS: MANUFACTURING, PROCESSING & DISTRIBUTION
Positron is negotiating a strategic alliance with Jubilant DraxImage Inc. (JDI), an Sr-82/Rb-82 generator manufacturer, whose generator and related infusion cart is currently in the FDA approval process. Upon FDA approval, Positron expects to market and distribute the first and only alternative to Bracco’s Cardiogen-82 generator. Positron will supply JDI with Sr-82 for their generator production. Positron intends to offer generators to their current and future Attrius customers and will introduce new customers to JDI’s generator utilizing Positron’s current nuclear cardiology network. Initial efforts will be focused on North America.
Also, as per management’s concall comments ,they have appointed distibutor for RUBY FILL for European Economic Area (EEA).
So, not able to give exact no. but should get rough idea I hope…
Today’s ET Article:
SEC Suggests Approval for Remdesivir in Tablet Form
New Delhi:
Remdesivir, an antiviral used for treatment of Covid-19, may soon be available in tablet form as a top panel of the country’s drug regulator has recommended its emergency use authorisation (EUA), people in the know told ET.
The Subject Expert Committee (SEC) of Central Drugs Standards Control Organisation (CDSCO) in a meeting on Friday recommended EUA for remdesivir tablets proposed by Jubilant Life Sciences, they said.
“The drug regulator will soon take a decision regarding the same,” a government official said.
Remdesivir is currently administered in the country through intravenous route.
Jubilant is planning to launch 20 mg sub-lingual tablets of remdesivir, sources said.
Sub-lingual administration involves placing a drug under the tongue so that it gets dissolved and absorbed into the blood through the tissues.
An email sent to Jubilant Life Sciences did not elicit any response till press time Tuesday.
Remdesivir is already removed from the Covid protocol regime.
So far it is still in use.
EU too continues to use it.