Summary of Mankind’s RHP -
SUMMARY
Mankind Pharma was incorporated in 1991 by promotors Rajeev Juneja, Ramesh Juneja & Sheetal Arora. The company is engaged in manufacturing & marketing diverse range of formulations and several consumer healthcare brands like manforce, prega news etc. Although having entered consumer healthcare only in 2007, ManKind has managed to be category leader in male condoms (ManForce), pregnancy detection kit (prega news), & emergency contraceptive pills (unwanted 72).
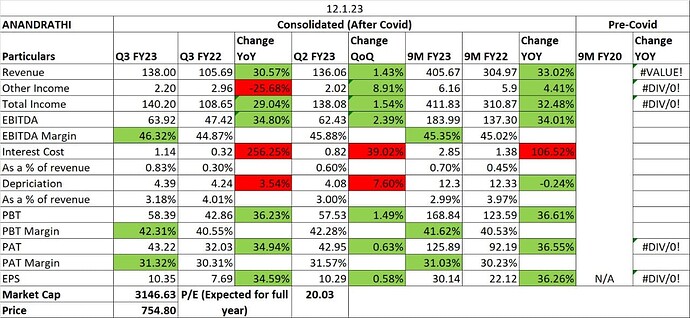
If one calculates with the FY22 reported numbers, the business is generating ~70% Gross Margins, ~28% EBITDA Margins, and is generating RoCE [EBIT(1-Tax)/ (Total Debt + Total Equity)] of 22%. Its earnings have been on an increasing trend & have grown at 18% CAGR from FY15 to FY22. However, investors investing in this issue should be mindful of certain points discussed below, that might have artificially increased the return rations.
Earnings for 9MFY23 have been lower compared to 9MFY22. At the upper price band of Rs 1080, its post issue implied market capitalization would be ~Rs 43266 crores and its asking valuation is ~32x FY23E PE.
Although pharmaceutical business is highly competitive in India, many of the players are making good returns on capital employed. Mankind’s asking valuations is at par with other listed players with similar returns profile. Currently, the gray market premium (“GMP”) is around 10%, which is indicating muted listing expectations from this issue.
Entire IPO is offer for sale wherein up to 2.5% stake would be sold by promotors & remaining would be sold by early investors such as Cairnhill & Beige. Around ~97.6% revenue comes from India operations & as per the management, this would continue as they are upbeat about Indian Pharmaceutical Market.
An investor investing in this issue should be aware of the below mentioned points –
- There had been impairment of loans given to related parties – Casablanca Securities & Indu Buildwell Pvt Ltd amounting to Rs ~17.7 crores in FY21 which was later reversed in FY22. However, going forward an investor should consistently follow the “loans to related parties” account.
- There is a negative capital reserve of ~909 crores created due to several acquisitions such as Relax Pharma Pvt Ltd, Medifore Healthcare Pvt Ltd & others. This negative capital reserve has artificially increased the return ratios to some extent.
- Inventory as a % of COGS has increased to ~73% in FY22 from avg of ~50%.
- Although not significant, one of the members of the promotor group Greesh Juneja has not provided his consent to be identified as the Promotor Group.
MANAGEMENT
Ramesh Juneja is the promotor & founder of the company with over 31 years in the Pharma Industry. He is the chairman & the whole-time director.
Rajeev Juneja is also the promotor of the company with over 29 years of experience in the Pharma Industry. He is the Vice Chairman & Managing Director and has been associated with the company since 1992.
Sheetal Arora is CEO & whole-time director of the company. He is also the promotor & is associated with the company since 2007.
INVESTMENT THESIS
Mankind’s track record provides comfort on execution capabilities of the management. Keeping aside huge success that they have had in scaling consumer healthcare brands such as manforce, prega news etc., one notable execution is when they launched their synthetic hormonal drug “Dydroboon” in 2019. Prior to mankind’s entry into this category, “Duphaston” the signature drug of Abbott India used to dominate the market. However, within only ~2.5 years of entry, mankind’s Dydroboon has now captured market share of close to ~22%.
19 brands out of their 20 best selling brands are ranked amongst the top 3 in their respective molecule groups. The company also has one of the largest on ground pan India marketing presence with a field force of 11691 medical reps, & 3561 field managers. Due to the sheer size of on ground reps marketing mankind’s products, it is likely to aid faster adoption of all new products that the company launches. Examples include – when they leveraged their existing presence for faster rollout of a Vildagliptin & Dapagliflozin tablets.
As per the details furnished in the RHP, Mankind’s manufacturing facilities are fairly underutilized & therefore going forward, there shouldn’t be significant capex towards setting up new facilities. Higher utilization would also allow room for operating leverage to kick in.
As of Dec 2022, Mankind was the most prescribed pharmaceutical company in India having ~15% share of prescriptions. Having a leadership position in prescription creates a circular network effect, where doctors prescribe partly based on what they believe pharmacists stocks, and pharmacists in turn favor brands that they believe doctors will prescribe or that patients would prefer.
Management guides on increasing their market presence in Chronic Therapies such as hypertension, diabetes, cardiovascular diseases. Company has filed 1 INDA (Investigative New Drug Application) for a novel G protein coupled receptor target for treatment of type 2 diabetes which is in phase 1 clinical trials. Increased presence in Chronic Therapies modes well as chronic therapeutic patients have a higher lifetime value. As they are planning on increasing their presence in Chronic Therapeutic areas, they have acquired one dermatology brand “Daffy” & one respiratory brand “Combihale” from Dr Reddy’s in Feb 2022.
They manufacture their own API for certain of their own products which includes their flagship products such as Telmikind & Dydroboon. This provides vertical integration which helps in maintaining margins.
ANTI THESIS
Regulatory & Compliance risk is a risk which is inherent to almost entire Pharmaceutical Industry. One cannot mitigate this risk however it is very important for investors investing in this issue to keep in mind the risk of bad observations by USFDA & other regulatory bodies.
The entire issue is Offer for Sale (OFS) wherein the company would not receive any monies from the proceeds of the IPO.
As of Dec 2022, products manufactured by third party manufacturers contributed to ~25% of entire revenue from operations. This includes medicines such as Entromax Suspension, Electrokind – L Liquid, Racigyl – SB Sachet etc.
Although currently only ~17% of Mankind’s products are under National List of Essential Medicines 2011 (NLEM), if there is any increase by the government, this would impact profitability & pressure margins. As of now, company’s 213 products are listed on NLEM, any further price caps from the government would impact margins.
Cash conversion days has increased from 106 days in FY18 to 155 days in FY22.
| Particulars |
2018 |
2019 |
2020 |
2021 |
2022 |
| D/E Ratio |
0.14 |
0.10 |
0.04 |
0.05 |
0.14 |
| EBITDA Margins |
22.0% |
19.6% |
26.4% |
29.5% |
28.3% |
| Gross Margins |
66.2% |
65.4% |
68.0% |
71.3% |
68.9% |
| Net Profit Margins |
13.9% |
12.1% |
17.8% |
20.8% |
18.7% |
| Post Tax RoIC |
27.1% |
25.2% |
34.9% |
33.8% |
26.9% |
| Post Tax RoCE |
21.7% |
20.8% |
29.6% |
26.5% |
21.5% |
| Before Tax RoCE |
31.7% |
29.4% |
40.1% |
34.6% |
29.0% |