Indoco Remedies Q4 concall highlights -
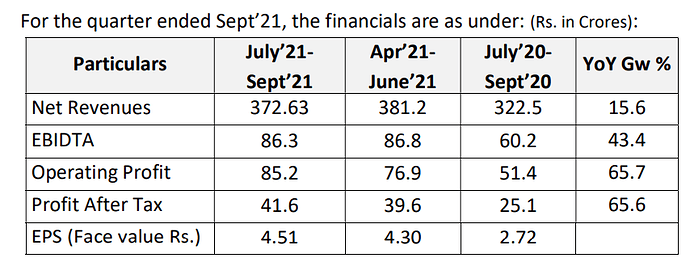
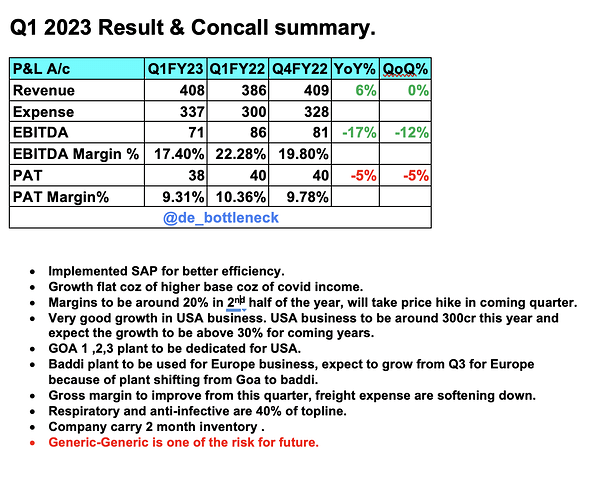
Revenues - 428 vs 409 cr
EBITDA - 65 vs 81 cr
EBITDA margins at 15 vs 20 pc
PAT at 30 vs 36 cr
For full FY 23 -
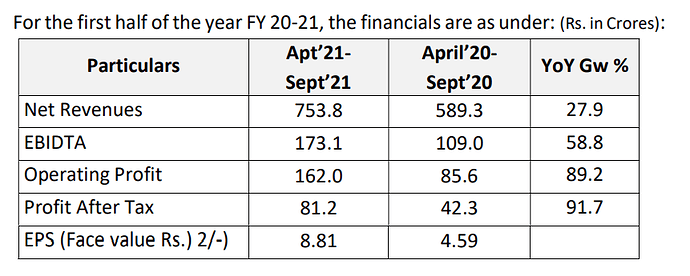
Revenues - 1669 vs 1541 cr
EBITDA - 286 vs 328 cr
EBITDA Margins at 17 vs 21 pc
PAT at 142 vs 150 cr
Base was very high due COVID related sales in FY 22
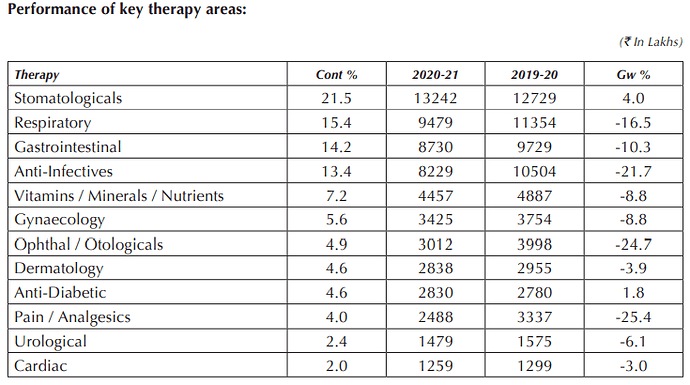
Domestic brand Cyclopam (used to relieve cramps) grew at a rapid pace
New product launches generated aprox 30cr sales
Top 20 brands of Indoco India generated double digit prescription growth
Two of company’s brands are in top 300 brands in IPM. Cyclopam jumped 27 ranks in FY 23
Company has three brands that generate > 100 cr and 04 brands that generate > 50 cr sales
Launched a D2C division this year for a few OTC dental brands
Plant-1’s import alert lifted by FDA
Plant-1 (solid dosage facility), now can export to US
In Europe, garnered 30 pc and 20 pc Mkt share for Lacosamide (to control seizures) tablets and Injections respectively
In Germany, company’s Allopurinol (to manage gout that causes intense joint pain) has 70 pc Mkt share
Business promo & marketing spends back to pre Covid levels
IPM rank at 27
Formulations-
Q4 India formulations sales at 185 cr, down 4 pc
FY 23 India formulations sales at 797 cr, down 1 pc
(India business had a big Covid base)
Q4 Intl formulation sales at 216 cr, up 14 pc
FY 23 Intl formulation sales at 754 cr, up 21 pc
Within Intl Mkts -
Q4 Revenues from regulated Mkts grew 5 pc to 163 cr
FY 23 revenues from regulated Mkts grew 21 pc at 610 cr
APIs -
Q4 API business grew by 75 pc to 23 cr
FY 23 API business grew by 12 pc to 71 cr
ndoco CRO operations-
Q4 revenues at 4.5 cr,down 5 pc
FY 23 revenues at 17 cr,up 12 pc
Base yr’s Covid sales in India were around 40 cr because of which company could not grow this year in the domestic market
Post clearance of Plant -1, US sales should also pick up next yr
Growth in advertising and sales promotion likely to moderate next year
Legal cost incurred this yr (aprox 5-6 cr) is unlikely to recur next yr
Company confident of doing 18-19 pc EBITDA for FY 24 and 25 ( that’s a descent jump from Q4 levels )
FY 24,25 growth expectations at 15 pc plus CAGR ( plus margin expansion )
US business has healthy order book. Likely to grow US business by 25-30 pc this yr
2.5 pc of domestic sales from new products (launched within last 2 yrs)
Expect the toothpaste business ( medicated ones ) to do better due to the D2C push initiatives that company shall take in FY 24
Attrition in MRs is an issue that company intends to resolve this year
Expect 3-4 approvals in US this yr - mostly in ophthalmic space
Expect a price hike of 5-6 pc in domestic mkt for FY 24
Company has overpromised and underachieved in the past 2-3 yrs. Mrs Parandikar was mindful of that. Intends to correct this in FY 24. The proof of the pudding will be in the numbers though
Ophthalmic and Injectables supplies to US involve very complex manufacturing practices. Company has been learning this over the last few years
Capex plan for FY 24,25 - aprox 125 cr
Disc: holding a tracking position. Hope to see an improvement in business momentum next year