They reduced the position from 5.23% to 2.73%
Granules Q2 FY25:
https://nsearchives.nseindia.com/corporate/GRANULES_06112024132254_NSEBSEINVESTORPRESENTATION.pdf
Disclosure: Invested
Management informed:
"This is regarding our communication dated September 07, 2024, about the US FDA
inspection of the Company’s facility at Gagillapur, Hyderabad, Telangana and the issuance of
Form 483 with 6 observations.
US FDA has classified the inspection as “Official Action Indicated” (OAI)."
Any inputs, from fellow VP members, on what could be the impact of this OAI on revenue / profitability.
Disc: Not Yet invested, but Tracking from quite some time.
Official Action Indicated (OAI) is a classification given by the US Food and Drug Administration (FDA) to a facility that is not in compliance with current good manufacturing practices (CGMP). The FDA notifies the company of the classification within 90 days of the inspection.
An OAI classification can mean:
- A facility has been cited for egregious violations related to food safety
- A facility has an uncorrected Voluntary Action Indicated (VAI) condition from a previous inspection
- A facility has a condition that poses an imminent health hazard
- The FDA may withhold approval of pending applications or supplements from the facility
An OAI classification does not immediately impact existing production or revenue, but it can lead to:
- Blocked new product approvals
- Further regulatory actions, such as warning letters or import bans
- Increased remediation costs
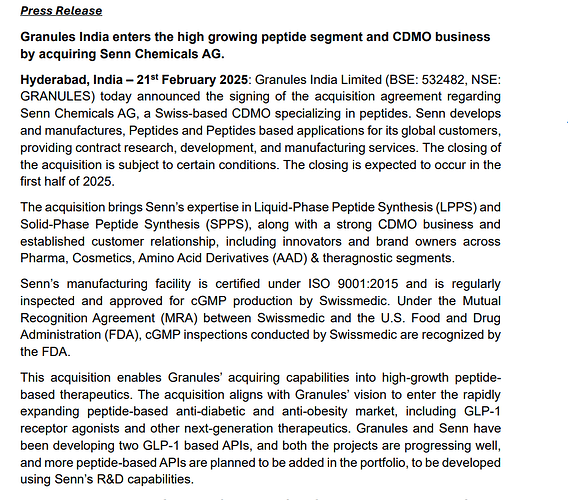
Finally Granules in moving into peptide CDMO by buying 100% of Senn Chemical AG for 192.22 cr
Q4 FY25: * Flat results from the company.
-
Revenue: ₹1,197.4 crores (+2% YoY).
-
Gross Margin: 63.4% (vs 60.1% YoY).
-
EBITDA: ₹252.4 crores (21.1% margin).
-
Net Debt: ₹706 crores (improved from ₹842 crores at start of year).
-
Cash-to-Cash Cycle: 202 days (vs 213 in Q3).
FY25 Full Year: -
Revenue: ₹4,481.6 crores (flat YoY).
-
Gross Margin: 61.5% (↑635 bps).
-
EBITDA: ₹945.2 crores (+10% YoY).
-
R&D: ₹238 crores.
-
ROC: 16.6%.
-
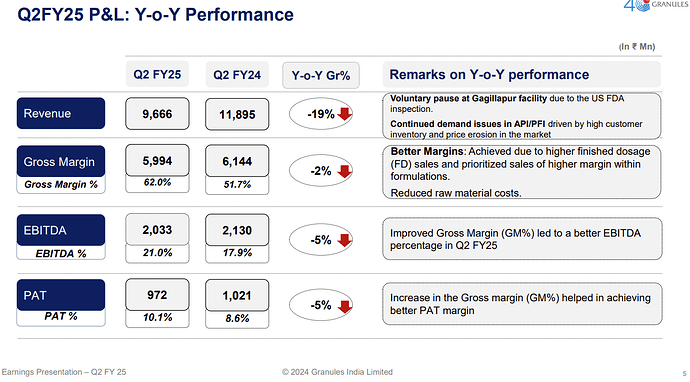
There is a major impact because of the FDA inspection at Gagillapur facility
Gagillapur FDA Inspection: -
August 2024: Inspected; six Form 483s issued.
-
February 2025: Warning letter received.
-
Manufacturing continues but with slowed operations due to remediation.
-
Over 1,200 batches and 2,600 swabs tested—no contamination found.
-
Remediation guided by three global consultants; expected to continue for 1–2 more quarters.
-
Remediation Cost: ₹60 crores in FY25; will continue at a lower level in H1 FY26.
-
For a Company of 12K crore Market capital, it is investing ₹1000 crores in Capex of which 300+ is already spent and remaining to follow.
-
The company wants to pivot from API, PFI manufacturing to FD, to move up in the value chain.
-
Looks like it is in transition towards with Peptides and oncology products.
Remediation & Operational Impact
- Remediation measures have impacted Q4 results and are likely to continue affecting performance for the next two quarters.
- Expenses: Incurred ₹60 crore at Gagilapur for third-party consultants, travel, and remediation in FY25.
R&D and Capex
- R&D Spend: Increased from ₹199 crore to ₹238 crore, a 20% rise year-on-year.
- Capex on Peptides: Investments ongoing in both Switzerland and India, targeting large-volume peptide products.
- Overall Capex Target: ₹600 crore, including investments in Seen Chemical.
Subsidiaries and Strategic Moves
- Seen Chemical: Currently at or near breakeven. This situation may persist for another 2–3 quarters, after which a new strategy will be implemented.
- Innovator Targeting: Focusing on some innovator products. High-volume GL1-related products are likely to be manufactured in India.
Sales & Product Pipeline
- Europe Sales: Recently driven by Paracetamol API. However, future growth will focus on formulation launches, which were delayed but are expected to proceed going forward.
- Oncology Platform: One product filed; approximately 10 more in the pipeline. This segment is expected to start contributing meaningfully from FY28 onwards.