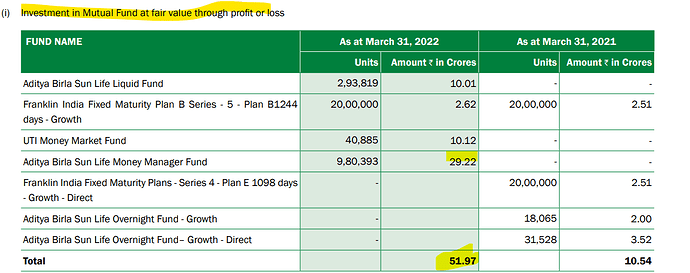
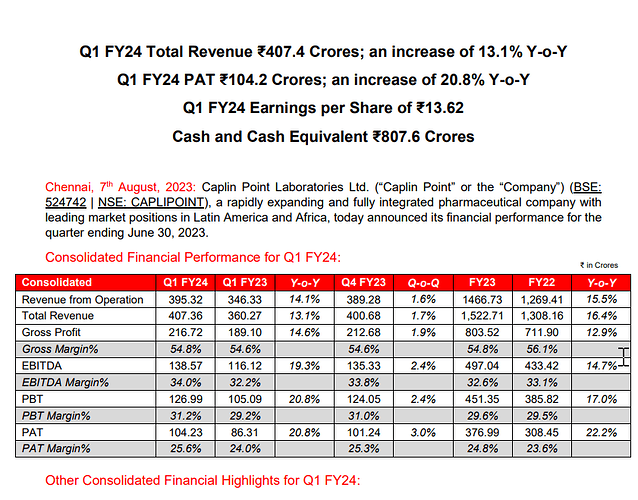
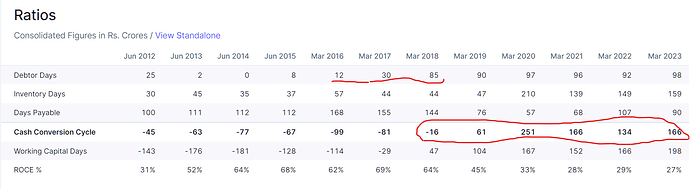Q4FY23:
• Caplin Steriles(US Injectable business) Total Revenue crosses Rs. 213 Cr in FY23 with 67% YoY growth; achieving PAT breakeven.
• Geographical breakup of sales: LATAM & ROW - 86%(PY:90%), US – 14%(PY:10%)
• Softgel expansion at CP-1 plant completed, with commercial exports commencing in Q4
• Company plans entry into Brand Marketing at Latin America, specifically in CNS and CVS segments.
• Development of 65+ API’s both in General Category and Oncology completed at R&D scale, to be scaled up when Company’s API units go on stream in the next few months.
• Company’s exports to newer markets such as Cambodia, Turkmenistan and Uzbekistan has commenced in Q4FY23.
• Company has received 2 tender awards from a LatAm country for Speciality & Oncology products, to be delivered in Q1 and Q2 of FY24.
• API Facility:
a. General Category API site refurbishment work ongoing, company targeting completion within 4 months.
b. Oncology API site construction starting in adjacent facility to the Finished Dosages Oncology plant at Kakkalur, Chennai. Targeting completion latest by Q3FY24.
• Capacity Expansion at CP-1 (ROW facility) – Softgel capacity expansion completed, with 2x the current capacity established for existing markets. Injectable expansion ongoing – lyophilization capacities to be expanded by 4x.
• - Capacity expansion in Caplin Steriles:
o Phase 2 of the facility nearing completion, commercial batches targeted by Q3FY24 from this unit. Post completion, company will be able to leverage large batches with faster filling speed for Injectable Vials. Also, an automated Pre-Filled Syringe line is being added, a new delivery system previously not available at Caplin Steriles.
o Phase 3, a standalone plant close to the current site is expected to be completed within Q4FY24, which will have high Lyophilization capacity, and plans to add complex dosage forms such as Inhalations.
• Oncology Facility – Oral Solid Dosages nearing completion. Injectable phase to be completed within 9 months.
• OSD Facility for Global markets – Construction work to commence shortly on a new Oral Solid Dosages plant in Thervoy SIPCOT, near Chennai. The facility, which is expected to be completed in 12 months, will increase existing OSD capacity by 3x and will cater to additional demand from larger LatAm markets such as Mexico and Brazil, in addition to regulated markets such as US and EU.
• CAPLIN STERILES: With a healthy order book, Company targets 50% growth in revenues in the FY24. Increase in revenues targeted through new product launches and higher market share from current products.
• Company has 8 ANDAs under review with FDA as on date, which includes Injectables and Ophthalmics.
• Company has completed 4 complex products Exhibit Batches, which includes 3 Injectables and 1 Ophthalmic. Plans to file all 4 with US and Global markets during FY24
• Company has launched its co-labelled product in the US, for 4 approved products.
• Overall development pipeline remains robust, with 55+ ANDAs under development with an addressable market in US at over $5 Billion.
• Company has earmarked Mexico and Chile as the next immediate avenues for growth in LatAm. Company has 1 product approved in Mexico, with 6 more approvals expected in the next few quarters. Company currently has 75 product registrations in Chile.
• Planning to have front-end presence by H1FY24 in the US market to launch own label & expand.
CONCALL NOTES:
• Growth drivers for FY24: One, our additional line of Softgel we produce a business of INR80 crores to INR90 crores from LatAm markets for the current year. Two, the brand marketing that we started in Central America, that is LatAm would also fetch us a revenue of INR30 crores to INR40 crores. Three, the introduction of generic business in West Africa might also result in an additional revenue of INR10 crores. You are aware that we are currently doing only brand marketing in this part of the world.
Four, our new initiatives in CIS and Southeast Asia could also fetch us a business of INR20 crores to INR25 crores for the current financial year. Five, Caplin Steriles would generate an additional income of INR90 crores to INR100 crores for the current year.
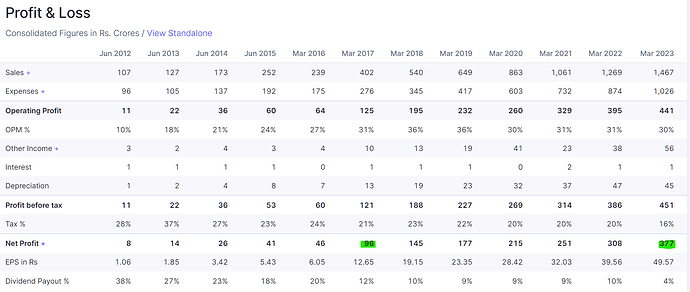
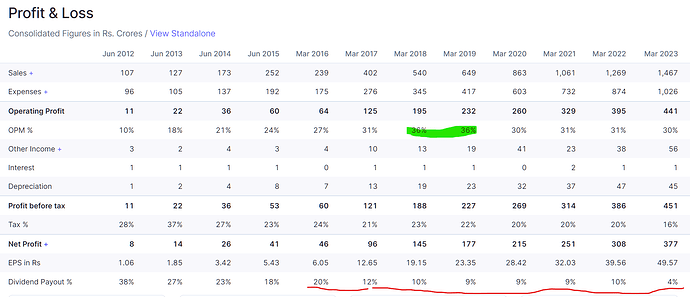
Overall 250cr sales growth. (17% over FY23)
• We are, of course, very happy with the progress Caplin Steriles has made in the last financial year, where we have grown nearly 70% and also achieved the full break even with a small profit as well. We need to also take into account that this includes all of the expenses that we incur in the way of ANDA filing, the R&D cost, the regulatory costs, etcetera which I believe amounted to close to INR20 crores, which is a direct hit and now specifically most companies I believe capitalize these expenses, whereas we charge them off with expenses. So, achieving the break-even despite a large regulatory and filing fees is something that we can all be appreciative of.
• CAPLIN STERLIES: We are sitting on an order book of close to INR230 crores to INR240 crores as we speak. So, the key is to make sure that we execute the production with high degree of compliance like what we have always maintained. The focus right now is to complete the validation of our new line, especially the ones that Chairman explained, which is high-speed and highly compliant line that we’ve bought from Bosch. Now we feel that this is going to significantly reduce our turnaround time. It will significantly increase our productivity as well. We are hopeful that this should go onstream by October of this year and that will free up quite a bit of capacity for us to continue doing our R&D work and exhibit batches work and stuff, which is the last thing that we need to do before filing the ANDAs.
• In terms of market share, for a majority of the products that we have live in the market, we have increased our market share
• But of course, the most important thing for us is to make sure that our business model is differentiated because that is the one that is going to be impactful over the long run and that is what is going to make it even more sustainable. This should happen once we start making more regular visits to the U.S., which will start in the coming couple of months onward.
• As a smaller company, we would like to believe that over a longer broader portfolio, we would have a good hold on the COGS. And once we go for backward integration on a few of these products, we will have even more control on COGS as well. So, we can be certainly encouraged by what we would see in the market on these products.
• “There are only two things that are permanent in the world, one is change and the other one is poverty. As long as you cater to the bottom of the pyramid, by removing the intermediaries and the bottom of the pyramid business itself will become a creamy layer and that is what actually has helped the company to where it is today. So, we’ll continue to do the same business, we’ll try and do the same thing in the regulated markets, ROW and the bigger geographies. Only difference is it takes time. It’s not like actually the ROW market where the registration gets completed in six months, their own places where we have studies, their own in fact actually expect the kind of dossiers and the regulatory hurdles that we have in the biggest geographies is not there. However, we will continue to grow in this domain and we will also go for something long term in the regulated markets where the big boys are ruling currently.”
• MEXICO: The model that we will be adopting in Mexico definitely is going to be very unique. But again, Mexico is a market where the registration and other things it takes long time. And now we are in the process of registering our injectables towards there and we will also get into OSD and other product shortly. Once we get a basket of actually 50, 60 products with different buckets in the form of tablets, capsules and injectables, probably we’ll start our business. We are doing actually a bit small business, not a big business, the consistency will start, say, one to two years from now in a big way, maybe two years from now, that would be the right timeline.
• BRAZIL: We are still in the registration stage. Here, the arrangement is totally different, it’s not like continue, which we do in Central America in the form of our work. We have a partner; he is registering the products. Again, it will take actually as we told you right now, Mexico, it will be still more, maybe definitely not less than two years.
Okay. So, both of these markets will only significantly fall into sales in two years
• Of course, I mean, the thing is, touch wood, we have filed by ourselves around 25 products and we have filed another five with partners. We have not even received one refusal so far, we have not had any product that has taken more than 18 months to get approved, right? So, we have an excellent track record for a company that is very new in this space.
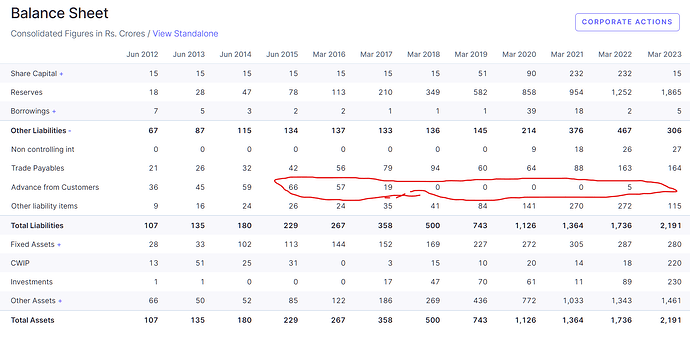
• CAPEX DETAILS: Yes, what is visible as of today is the ongoing CSL project, Phase II and Phase III, which will consume anywhere between INR150 crores and INR170 crores. The Onco project will consume another INR50 crores, INR60 crores. And as Chairman was mentioning, another OSD plant, greenfield plant is being initiated this year. The total outlay is about INR150 crores, I’m not sure how much of that will get consumed within this year. Between FY24 and H125 anything between INR350 crores and INR360 crores will be capex as planned today.