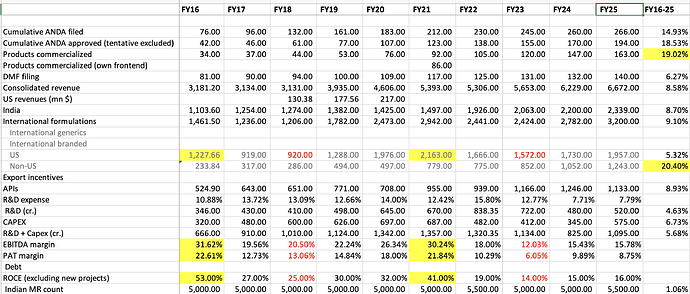
Alembic Pharma -
Q2 FY 25 results and concall highlights -
Revenues - 1648 vs 1595 cr, up 3 pc YoY
Gross margins @ 74 pc
EBITDA - 257 vs 218 cr , up 18 pc YoY ( margins @ 15.6 vs 13.8 pc )
PAT - 153 cr, up 12 pc YoY
Segment wise sales -
India formulations - 609 vs 577 cr, up 6 pc ( 18 pc of India business ie 118 cr comes from Veterinary formulations ). Alembic is ranked no 20 in IPM. Company has a MR strength of 5200 MRs. Company has 4 brands with sales > 100 cr / yr. Veterinary business grew by 20 pc in Q2
US formulations - 467 vs 444 cr, up 5 pc. 8 products launched in US in Q2. 10 more launches are lined up in H2. Products from new facilities are expected to drive future growth. Capacity expansion is underway in the Oral Solids plant to meet the immediate demand in US and other export mkts
Company has a total of 214 ANDA approvals. Have launched 157 products so far. Company has 16 Injectable, 30 Derma, 19 Ophthalmic product approvals. Rest are oral solid approvals
Company aims to increase the complexity of products launched in US in order to obtain better pricing
RoW formulations - 298 vs 252 cr, up 18 pc. Mainly operating in EU, Canada, Aus, Brazil, RSA and Chile. Future growth to be led by product and geography expansion. Have line up expansions in ME, NA and Mexico
APIs - 274 vs 322 cr, down 15 pc
( mainly because of price erosions and the high growth base that the company has in this segment )
Manufacturing footprint -
Formulations facilities - 05 ( three of these have had USFDA audits in last 6 months, including the Onco Injectables facility )
API facilities - 03
US mkt is seeing good volume growth backed by recent launches. Same should continue in H2 as well as a number of new launches are lined up in H2 as well. However, price erosion in US mkt continues @ high single - low double digits
Acute business in India de-grew by 2 pc in Q2. Therapies like Gynae, Anti-Diabetic, Opthal grew by 8 pc, 11 pc, 18 pc and 13 pc respectively
Gross Debt @ 995 cr
Cash on books @ 125 cr
R&D expenses @ 8 pc of sales
Expect continued growth momentum in RoW business
Expect to clock a high single digit sales growth in India business in H2. Company expects H2 to be much better than H1 for US and RoW business
R&D spends for full FY should be around 500 cr
Expect API business to start reporting growth wef FY 26
In the RoW mkts in Q1, company had some supply chain issues. The same are now behind, which resulted in good growth in Q2. Expect H2 to maintain similar growth trajectory as Q2 for RoW business. In the US, expect a much better H2 led by new launches and ramp up of launches made in H1
API business is also seeing aggressive price erosions. Chinese aggression is a part of the reason
Company is in partnership with Natco Pharma to launch Olaparib ( Onco Drug ) in US. Have not yet arrived at a settlement with the innovator - AstraZeneca
Higher inventory levels at the end of Sep 24 are because of supply chain issues that the company faced in Q1 and hence they did build up inventory to offset those issues. They also expect much better H2 business in US + RoW mkts. This inventory will hence normalise going forward
Company is setting up a new formulations facility near Indore for the domestic mkts. That should come up by end of FY 25. Additionally, company is adding peptide manufacturing blocks to one of their API facility. Plus they are adding 2 more lines in their F3 formulations facility. These are the works in progress
Have filed for 2 peptides in the US mkt. These are not GLP-1 products. However, the company has ambitions to make both peptides and formulations for GLP-1 products ( for both Semaglutide and Tirzepetide ) and are working towards them. Most likely, company will be among the second wave of generic players launching these products. Have been doing R&D on peptides for 4-5 yrs now
As the inventory levels reduce in H2, the gross debt levels should come down by end of the year
Disc: holding, biased, not SEBI registered, not a buy/sell recommendation