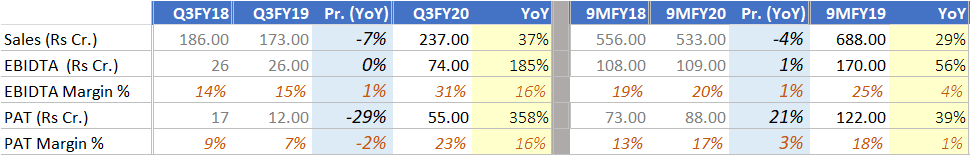
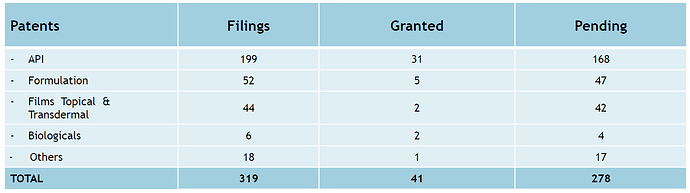
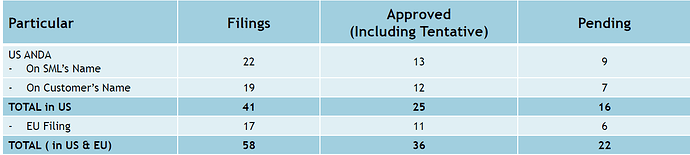
This drug was awarded as orphan drug with exclusivity up to 15 Oct 2021. This approval is tentative. Final approval will come once patent expires or court order is to cancell the patent
Positive : FTF from Shilpa
Negative : Long way to go on patent expiry front (a portion of copy of letter sent to Apotex by USFDA)
The RLD upon which you have based your ANDA, Hoffmann’s Esbriet Tablets, 267 mg and
801 mg, is subject to periods of patent protection. The following patents and expiration dates
are currently listed in the Agency’s publication titled Approved Drug Products with Therapeutic
Equivalence Evaluations (the “Orange Book”):
U.S. Patent Number Expiration Date
7,566,729 (the '729 patent) April 22, 2029
U.S. Food & Drug Administration
10903 New Hampshire Avenue
Silver Spring, MD 20993
www.fda.gov
ANDA 212709
Page 2
7,635,707 (the '707 patent) April 22, 2029
7,767,700 (the '700 patent) December 18, 2027
7,816,383 (the '383 patent) January 8, 2030
7,910,610 (the '610 patent) January 8, 2030
8,013,002 (the '002 patent) January 8, 2030
8,084,475 (the '475 patent) January 8, 2030
8,318,780 (the '780 patent) January 8, 2030
8,383,150 (the '150 patent) September 22, 2026
8,420,674 (the '674 patent) December 18, 2027
8,592,462 (the '462 patent) April 22, 2029
8,609,701 (the '701 patent) April 22, 2029
8,648,098 (the '098 patent) January 8, 2030
8,754,109 (the '109 patent) January 8, 2030
8,778,947 (the '947 patent) August 30, 2033
9,561,217 (the '217 patent) January 25, 2022
10,188,637 (the '637 patent) March 28, 2037
1548388128266728.pdf.pdf (181.3 KB)
Manoj,
Can you go through it. It was given an orphan drug status till 15 Oct 21. Now it is under jurisdiction. Many companies have filed cases against patents. As per my limited understanding, it will be difficult for orphan drug to defend patents in court.
Also Shilpa has applied as NCE claiming it bioequivalent.
That is correct on orphan drug status. Yes many companies involved in this so called patent infringement. Not sure how this can pan out. Even Apotex has claimed it as bioequivalent & has satisfied FDA on clinical trials (as opposed to what Shilpa medicare has done-as per the attached case docket).
This is not for Shilpa. I have attached the link
Investor presentation has nothing special. AR has many things to understand. I observed only one point in IP that biologics plant 1st phase is going to commission in Jun 20 in Dharwad. Without human trials and 505b2, it is nothing. But it gives hope that they are near to crack. As per AR timeline, they should be near to crack it. As a way forward I consider Shilpa’s entry into biologics and Novel drug delivery a game changer.
Disclosure - Large portion of my portfolio. No buy or sell in last 3 months.
Giving my viewpoint on the 505(b)(2) development strategy of the company:
Blockquote
The company has taken a lead in filing for products under the 505(b)(2) route, which is a capital intensive process and requires tremendous R&D capabilities. Considering the potential complexity and risk, payoffs in a 505(b)(2) molecule are manifold in comparison to a traditional ANDA filing.
Blockquote
Folks, this is to sensitize you about the marketing risk of 505(b)(2) products. I repeat marketing risk (not development risk).
ANDA Applications
- ANDA applications [505(j) of the FD&C Act] are always filed with respect to an existing approved drug - RLD (Reference Listed Drug) and RS (Reference Standard).
- An approved ANDA application gets a Therapeutic Equivalance belonging to A* category ( AA , AN , AO , AP , AT, or AB) rating assigned by the FDA. Drugs with these TE ratings are substitutable at pharmacy level. So if the prescription says “Celecoxib 300 mg capsule”, then the pharmacy can give any of the approved ANDA formulations of Celecoxib that it stocks.
- So the market for an ANDA product already exists. The company’s sales will depend on the no. of competitors [which determine the Market Share and Price Erosion levels].
505(b)(2) Applications
- Now coming to 505(b)(2) products. There is NO established market for the 505(b)(2) product.
- For example search FDA Orange Book for Desvenlafaxine. You will see two Desvenlafaxine products (50 mg and 100 mg) having application no. [N204150].
- This was Alembic Pharma’s 505(b)(2) of Desvenlafaxine. Launched sometime in 2013 (while the generic Pristiq launch was still away - around 2017). Blockbuster sales of Desvenlafaxine 505(b)(2) were anticipated BUT nothing of that sort happened.
- Because to sell 505(b)(2) in market, the company needs (i) Doctors to write prescription for the 505(b)(2) product [pharmacy cannot substitute it for the Innovator or any other Generic of the Innovator] (ii) Have the product in formulary list of Insurance companies / PBMs.
- Both these require Sales & Marketing field force who run from pillar to post to make this happen. In contrast, sales of ANDA drugs does NOT require any sales/marketing field force. Only skeleton staff which manages contracting and price negotiations with the Wholesalers, GPOs, PBMs, other significant buyers in the market.
So as a matter of sound commercial prudence, neither the company, nor its investors should under-estimate the marketing risk of 505(b)(2) products.
FDA has a Guidance Document on which pathway to choose. It can be referred to understand technical differences.
Quoting a para from FDA document:
A scientific premise underlying the Hatch-Waxman Amendments is that a drug product approved in an ANDA under section 505(j) of the FD&C Act is presumed to be therapeutically equivalent to its RLD. Products classified as therapeutically equivalent can be substituted with the full expectation that the substituted product will produce the same clinical effect and safety profile as the prescribed product when administered to patients under the conditions specified in the labeling. In contrast to an ANDA, a 505(b)(2) application allows greater flexibility as to the characteristics of the proposed product. A 505(b)(2) application will not necessarily be rated therapeutically equivalent to the listed drug it references.
I happened to look at the Annual Reports of Shilpa Medicare and came across a few observations that maybe useful to analysts / owners / prospective owners (one page attached)
I would love to have your thoughts or comments.
Warmly,
Kimi
Valuepickr-post-28042015.pdf (33.2 KB)
Valuepickr post 28042015.pdf (33.2 KB)
Attached. I had offered to respond to any observations members may have. Now that the post is close to 5 years old…that offer maybe considered withdrawn ![]()
It looks company wants to be visible now.
There are some other recently added corporate films too.
Shilpa Medicare Ltd gets 15 observations from USFDA for facility at Jadcherla
https://www.bseindia.com/corporates/anndet_new.aspx?newsid=f8dec7e0-58fb-4a92-a86b-b70f5f8f1b72
Any idea about the impact on its revenue?
Dear Sir/Madam,
Sub: Intimation U/R 30 of the SEBI(LODR) Regulations- Reg. Ref: Stock Code: NSE: SHILPAMED/BSE-530549
Shilpa Medicare Limited, through its UK subsidiary Koanaa Healthcare Limited, received UK MHRA approval for Imatinib oral solution.
The approval of Imatinib Oral Solution represents a major step forward in treatment options for cancers in pediatric and geriatric patients, patients with swallowing difficulties and also offers dose titration across the patient population.
Imatinib Oral Solution, a first of its kind in the world, is the only approved product which facilitates accurate dose titrations and avoids unnecessary overdosing due to fixed doses, 100 mg and 400 mg, in the presently available marketed tablets.
Imatinib Oral Solution will allow health care providers, oncologists and patients to jointly take control of their condition using a product with the same proven and safe mechanism of action as seen with currently available tablets.
Unlike current prescription treatments which are solid oral doses, Imatinib Oral Solution is ready solution, taken once a day or as prescribe, allows patients to adjust the dose according to patient requirements.
Imatinib Oral Solution is expected to be available in the UK in the second quarter of 2020 will be supplied from a UK based state of the art manufacturing facility LM pharmaceuticals.
Imatinib Oral Solution is an innovative product and is expected to have patent till 2038
Shilpa Medicare Limited Launches First Branded Gencric
Anticancer Drug - Dasatinib with all dosage Strength under
brand name “DASASHIL”
shilpa_announcement.pdf (1009.8 KB)
ab7b322f-34e2-4a2b-9d1c-e98726f2dca1.pdf (158.0 KB)
Shilpa is now ramping up on domestic business.
Future plan FY 2019-20:
• Shilpa has plans for filing one more first-to-file ANDA.
• Shilpa & it’s Partners has plans for filing of two 505(b)(2) NDAs.
• Shilpa plans to initiate Phase III trials of one biologic molecule.
In biologics
Where we are –
The company now has 6 biosimilars and one New Biological
Entity in its pipeline and is dominated by drugs catering to the
auto immune disorders and oncology segments, with 4 of the
top 10 biologics in its pipeline. The remaining are niche, high
margin opportunities catering to high unmet clinical needs.
Your company is forging ahead with clinical trials on 1 nos MAb, 1
nos fusion protein and 1 nos NBE during the course of the coming
financial year. The combined market size of these three drugs today
is about $30 billion. 3 more are expected to be added in the next
financial year to the Clinical trial pipeline, with market size of about
$13 billion. The revenues from sales of the first commercialized
biosimilar is expected to accrue from FY 2021-22 onwards.
The company has also filed 3 platform patents and is pursuing
these in global markets. Your company will pursue an aggressive
IP strategy to ring fence its biosimilar and NBE assets.
Your company is also expected to progress one of its
biologics into Human Clinical Trials designated as a New
Biological Entity (NBE) during the course of the coming
financial year. This is expected to be major revenue driver
from 2022 onwards – both, through direct sales and
licensing opportunities for the company.
Above are some excerpts from Annual Report 2019. Company is progressing with a big leap on biologics. No media coverage of Shilpa’s biologics plans. Already human trials are underway as per AR.
Five FTF filed and one FTF planned in FY20. I am waiting for AR to understand the progress.
One main point to note is that its subsidiaries should stop dragging profits as we have seen approvals in recent past throigh subsidiaries and some approvals to be sold through subsidiaries. It looks it is the smallest company working on so many fronts of specialty. Even Novel drug delivery started approvals.
Are they conducting any investor call post the results? If anyone knows, kindly provide details.