SML’s CDMO customer, Unicycive Therapeutics, Inc. has reported positive results from pivotal clinical trial of Oxylanthanum Carbonate (OLC), a NCE molecule.
SML has partnered with Unicycive to provide end-to-end CDMO services right from development & supply of APIs to finished dosage form.
Apart from the supply arrangement, SML is expected to receive US$ 10 million as milestone income spanning over filing, approval and launch of the product. Additionally, in anticipation of increased product demand Unicycive will also fund the establishment of new manufacturing block at Shilpa’s site.
A note on this CDMO product
First lets understand the basic of what is a phosphate binder (OLC is a phosphate binder) and what does it do
- In very simple words, patients with diabetes are sometimes not able to excrete the phosphate they consume during their meals, resulting in an elevated serum phosphate. This happens to patients with Chronic Kidney Disease.
- If your phosphate levels are too high, it can remove calcium from your bones, which makes them brittle. It can also cause calcium deposits in your eyes, lungs, heart and blood vessels, which increase your risk of heart attack, stroke and death over time.
- Binders are basically used to reduce the phosphate absorption, they need to be taken with each meal, and need to be taken lifelong.
There are 7 types of phosphate binder as of date
- Sevelamer carbonate
- calcium acetate
- sucroferric oxyhydroxide
- ferric citrate
- tenapanor
- lanthanum carbonate
- oxylanthanum carbonate (Unicycive’s molecule, under approval)
Sanofi is the market leader here, selling renvela (sevelamer), and has also licensed fosrenol (lanthanum carbonate) to Shire international.
- renvela is only sold in the US, while fosrenol is widespread generic.
Now why is OLC better then the other 5
-
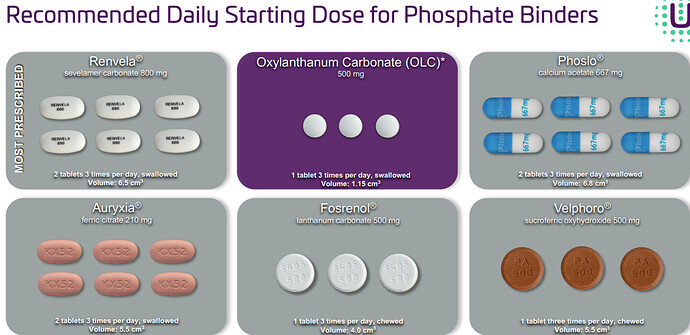
Patient compliance- according to the market research in US by nephrologists patient compliance increases significantly with reduced pill burden. The below shows the superiority of OLC. In very simple words, Unicycive has been able to develop some patented nanoparticle tech, through which they are shrinking the size of the pills.
renvela requires the highest number of pills to be consumed per day, but still is the highest seller.
Fosrenol although has similar effect, the tablet is bigger and with more AEs -
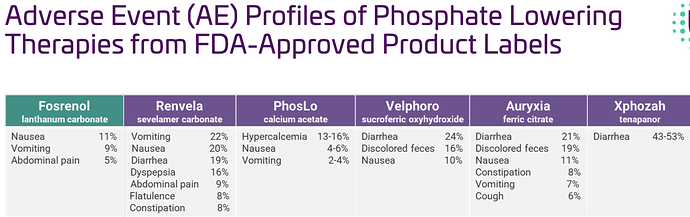
Adverse event profiles (AEs)- in simple words AEs are the side effects
The OLC treatment-related AEs reported in ≥5% of patients were diarrhea (9%) and vomiting (6%) which also compares favorably to Fosrenol and other phosphate binders on the market.
As can be seen from the above, again the top seller is way worse then OLC, even the rest are significantly higher then OLC. -
In terms of tolerability, OLC had a low rate of discontinuation due to adverse events (AEs) with only 2/86 patients (3.5%) discontinuing from the Study.
- The low discontinuation rate for OLC compares favorably to a discontinuation rate due to AEs of 14% for Fosrenol (the nearest competitor based on data)
-
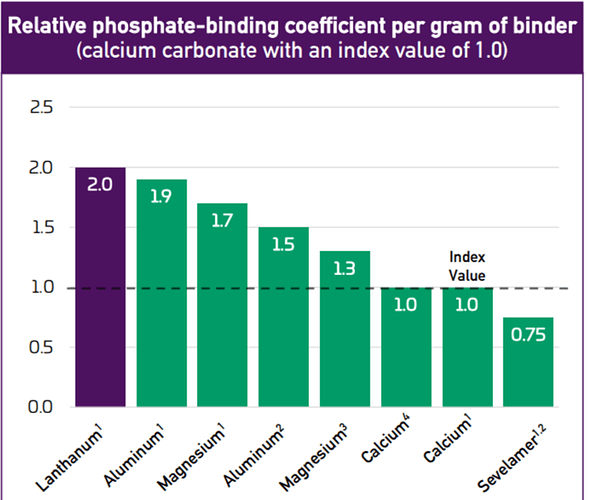
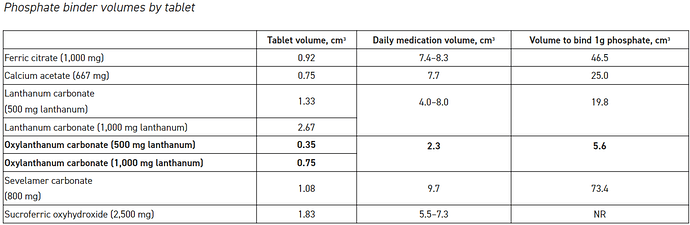
Phosphate binding coefficient- as i explained above, the binder absorbs the phosphate, so binding coefficient in very simple words is per gram of medication absorbing the phosphate content in the body. The higher the absorption the better
as can be seen from above, lanthanum (OLC) is able to absorb the highest volume of phosphate, while Sevelamer (renvela) is absorbing the least.
The above also qualifies Fosrenol as an effective medication as lanthanum is also used in this. -
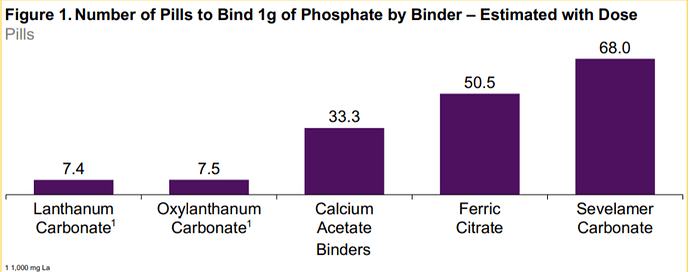
Oxylanthanum carbonate has the lowest daily phosphate binder dose volume and the smallest volume required to bind 1 g of phosphate compared to all other commercially available phosphate binders.
-
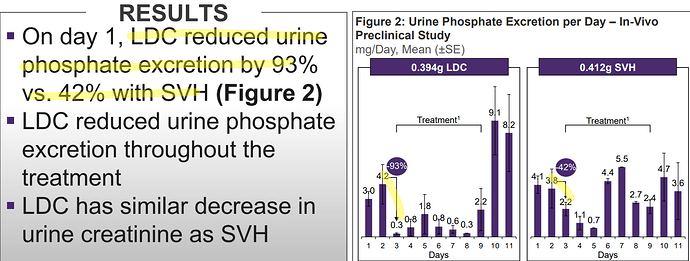
OLC versus SVH-
LDC is OLC, SVH is Selevamer
LDC had more reduction of urine phosphorus/creatinine ratio compared to SVH and control
Now a thing to be noted is that, even after availability of 6 FDA-approved phosphate binders, hyperphosphatemia remains uncontrolled in an estimated 75% of US dialysis patients.
Maybe this is because of lower patient compliance, as explained above, or these medications aren’t as effective, or that this disease or illness isn’t much cared about.
The only one comparable to OLC is Fosrenol, it is comparable across factors like the no.of tables to be consumer per day, the AEs, the volume required, the absorption.
- need to understand how will they upsell OLC against fosrenol, which being a widespread generic i would assume will be very cheap to OLC.
As we saw above Fosrenol is a lot better then the other binders (excluding OLC), still renvela is the top selling product and Fosrenol doesn’t seem to have been picked up much, this raises doubts as to if fosrenol couldn’t switch renvela, why will OLC.
- i am really not able to understand why is renvela the top seller, with so many inefficiencies and issues.
As of today’s call, the US market alone is 1 billion dollars, and from the data shared above we can clearly see the superiority of OLC against other binders, hence one would expect a quick turnover of products. This also somehow reflects in the confidence of Unicycive, with them willing to fund a whole capex worth $6.5 million with Shilpa, with a contract consisting of binding volumes for 4years
- something more around the total market- approximately 40% of patients on dialysis are unable to achieve adequate serum phosphate control as defined by established KDOQI treatment guidelines1. The UNI-OLC-201 study was representative of the U.S. dialysis patient population.
the above data is from the clinical research data of Unicycive and some other sources.
invested here since lower levels, hence biased
Sucessfully completed the Phase 1 clinical trials for rHA (Recombinant human albumin)
69b044ba-abc8-4379-866f-a82693d60056.pdf (625.8 KB)
Subject :- Shilpa Medicare announces successful outcome of Phase 3 studies of SMLNUD07 – NorUDCA
Shilpa Medicare has achieved a significant milestone with the successful completion of Phase 3 clinical trials for its novel product, SMLNUD07 – Nor-Ursodeoxycholic Acid (NorUDCA) tablets, a potential game-changer in the treatment of Nonalcoholic Fatty Liver Disease (NAFLD). This trial, which was a multicentric, placebo-controlled, double-blinded study, involved 165 NAFLD patients across India, providing a robust and statistically reliable dataset. The study demonstrated that NorUDCA was well tolerated at a dosage of 1500 mg per day over a 24-week period, with no serious adverse events reported. Efficacy results were promising, with patients experiencing at least a one-stage reduction in liver fibrosis, as measured by the USFDA-approved Fibroscan technique. Additionally, a significant reduction in hepatic fat accumulation was observed, evaluated using the CAP scoring technique. The study also reported a significant normalization of Alanine Aminotransferase (ALT) levels, indicating improved liver function. These findings suggest that NorUDCA could become a new standard of care for NAFLD, offering significant improvements in liver function and a holistic approach to treatment by addressing multiple disease outcomes.
Shilpa Medicare plans to submit these results to the Central Drugs Standard Control Organization (CDSCO) in India to seek marketing authorization. NorUDCA stands out as a first-in-class treatment option in India, with notable advantages over conventional Ursodeoxycholic Acid (UDCA), including enhanced choleretic effects, resistance to amidation, anti-inflammatory properties, and a reduction in fibrosis. NAFLD is the most prevalent liver disease globally, affecting approximately 25% of the world’s population, which translates to about 1.2 billion people. In India alone, around 188 million people are affected. If untreated, NAFLD can progress to nonalcoholic steatohepatitis (NASH), which can have severe and potentially fatal implications
Future seems promising for Shilpa also.
Shilpa Pharma Lifesciences Limited, Received CEP from EDQM for API, Desmopressin
Hi , can someone share the impact on the company of the announcement made today? Is this a key inflection point?
Shilpa Pharma Lifesciences Limited, Received CEP from EDQM for API, Octreotide
This is to inform you that the Shilpa Medicare Limited’s 100% subsidiary, Shilpa Pharma Lifesciences
Limited received certificate of suitability (CEP) from EDQM (European Directorate for the Quality of
Medicines & Healthcare) for API, Octreotide.
Octreotide is synthetic peptide manufactured by Shilpa Pharma Lifesciences through solid phase
synthesis. Acromegaly, severe diarrhea/flushing episodes associated with metastatic carcinoid tumors,
profuse watery diarrhea associated with Vasoactive Intestinal Peptide (VIP) secreting tumors.
Octreotide is second peptide molecule in Shilpa’ s peptide portfolio. The CEP showcases our expertise
and commitment to quality-oriented development and commercial production, which meets the
expectations of global quality standards.
Shilpa Medicare Q3 Highlights
- Revenue Growth: 11% YoY driven by strong performance in FDF and Biologics verticals.
- Gross Margins: 72% vs. 67% YoY, supported by a healthy product mix.
- Future Outlook:
- Strong traction from new product launches expected to drive revenue growth.
- Focus on scaling API business and launching approved NDAs in regulated markets.
- Prioritizing timely approvals for filed products and ongoing investments in the pipeline.
This is to inform you that the Shilpa Medicare Limited’s 100% subsidiary, Shilpa Pharma Lifesciences
Limited, Unit-1 received EIR from USFDA and the site is classified as VAI. The inspection was carried out
at unit-1 by USFDA between March 3-7, 2025.
What are they referring to cwip here since 2021, where are they spending that money?. I don’t see fixed assets or other assets increased in the same proportion as they mentioned in CWIP. Am I missing anything?
Fixed Assets 1,111 1,342 1,368 1,385 1,693
CWIP 541 506 655 719 455
Shilpa Medicare strategic partnership with Orion Corporation
Shilpa Medicare, has partnered with Finland’s Orion Corporation to out-license its recombinant human albumin for commercialization in Europe.
Payments will be in batches and marks Shilpa’s strategic expansion into global biopharma markets.