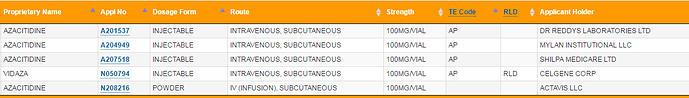
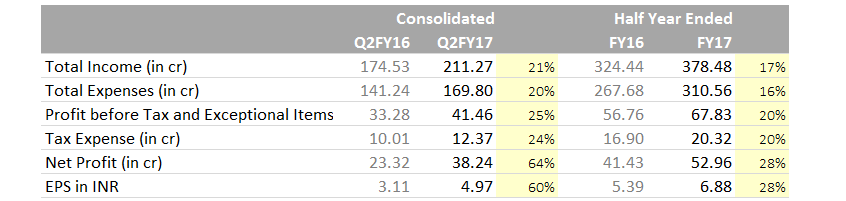
Hi Guys,
Apologies for the delay in uploading the AGM notes since it took a lot more time to compile them and time constraints from my end. The effort from the questionnaire to Q&A and then to compiling notes (not to forget the travel arrangements made by Hyderabad team) is a collaborative effort of VP team members who attended the AGM. The key points discussed are given below:
Key points of the the AGM 2016
Regulatory Compliance
Getting approval from PMDA, Japan (regulator for Japanese markets) is not very easy and it is considered to be amongst the most stringent regulators especially for oncology. They don’t even allow change in Ph, temperature or even spelling of name of different blocks. Its Japanese CRAMS partner guided the company in securing the approval for its API plant. The company is fully automating its systems and processes to reduce as much human interference as possible. Getting zero 483 observations from USFDA for oncology injectable plant given the current times is an achievement for the company. It has implemented software like Laboratory Information Management System (LIMS) for adhering to the guidelines of USFDA and other regulatory authorities. As per the software, automatic data can be generated and even USFDA audit members can access it without any human interference. It will give detail about stability data, acceptance/discard of a batch etc. The company is also implementing SAP currently to streamline the processes within the company. Data integrity is considered to be the major issue faced by most of the pharmaceutical companies for which Quality Control (QC) team plays a major role. The company has good QC team in place which ensures that they discard the whole batch which fails the test and company is ready to take short term hit on profitability on account of it.
Management Bandwidth
The company is building up a team of employees that ensures that Mr. Bhutada, MD, is not involved in the day-to-day operations of the company and delegates more. Mr. Bhutada has not visited the Vizag R&D laboratory for almost two months now. Most of these employees have grown from within the ranks of the company. They started from the lower levels and rose within the ranks of the company. The company rewards the employees and gives them chance to grow. The company is hiring employees from outside as well. Few of the employees hired for overseas subsidiaries like Koanna Healthcare and Loba Feinchemi have lot of experience in the pharmaceutical industry (details covered late).
API Business
API is the bread and butter of the company. The company is adding two more oncology blocks to take the total dedicated oncology blocks to 9. With these capacities, Shilpa’s API capacity for oncology will become one of the largest in India. The competition in the API segment for oncology is not much and has reduced substantially especially from China on account of clampdown from USFDA on the Chinese companies. The competition for APIs in oncology segment from China for US markets has reduced substantially on account of compliance issues. Currently, only two large Chinese companies are competing in these segment which are USFDA compliant. Although, there is decline in the prices of few of the key molecules like Capecitabine, however, the company is making up for the decline in prices by increasing the volumes. The company is planning to increase the capacity of Capecitabine to 2.50 times the existing capacity. When the company had increased the capacity to its current capacity for Capecitabine, there were doubts that how are we going to operate such large capacities but now it is almost running full. Even if we are not able to fully utilize the whole capacity after expansion, we should be able to tie up for 60 – 70% of the capacity by next year. There are insourcing risks by large pharmaceutical companies but they are certain molecules where we have expertise. Even though Intas has inhouse API sourcing for Capecitabine, it procures large quantity from us (in fact driving expansion in capacities) and the insourcing is primarily to derisk itself if Shilpa faces any regulatory issues in the future. Intas Pharmaceutical is the major customers of the company for its APIs and derives 60% of its API sales from it. We are not just dependent on few molecules like Capecitabine and Gemcitabine, but are manufacturing 16 - 17 molecules currently. Furthermore, we have lot many in the pipeline. We only select products where we have good margins which are expected to remain sustainable in the future even after price erosion. We believe in adding new blocks for new molecule instead of phasing out old molecules where we have high market share. We are even working on exhibit batches for molecules whose patent will expire in 2020 – 21 or even 2030.
API sales are dependent on formulations and thus the company forward integrated into manufacturing of formulations. Many of the DMFs filed by the company with USFDA are for molecules whose patent have already expired and it will be difficult to replace the existing API source for Shilpa in few of the molecules where regulatory approvals from USFDA will take time. The API sales in US will depend on new product approvals from which Shilpa is the API source as well as Shilpa’s ANDA filings from its formulation plant. Out of the 22 ANDAs filed for the US markets for formulations, 12 -13 have Shilpa as its API source while the rest will be procured from other sources. We wanted to derisk our formulation plant for its API source in case there is an regulatory issue in our API plant and hence not all the ANDA filed from the formulation plant will have Shilpa as its API source. The US API sales will majorly start from FY18 onwards. It is possible for cytotoxic manufacturers to also manufacture oncology APIs, however, many of the formulation companies prefer non cytotoxic facilities since it becomes difficult to segregate between cytotoxic and non cytotoxic molecules from the same blocks. Sometimes regulatory authorities do accept oncology molecules filed form non cytotoxic blocks of API companies but the formulation companies will not accept it. Human safety is more if oncology molecules are manufactured in cytotoxic blocks. The company also got Silver award for patent filing from Pharmexcil. Out of Rs.240-250 crore API sales during FY16, Rs.200 crore was through oncology molecules while remaining from non-oncology ones.
Formulations
The company has filed 22 ANDAs till date out of which 7 - 8 have been filed in its name while the rest have been filed in the name of customers for contract manufacturing. The contract manufacturing will be on ‘cost plus margin’ basis while the own ANDAs will have a profit sharing model with the partner. The formulation plant has approval from EUGMP (European markets), USFDA and ANVISA (Brazil). The company currently has two oral lines and two injectable line. The second injectable line is currently manufacturing exhibit batches for clients. The second injectable and oral lines were also covered during the USFDA inspection. The second line was set up in anticipation of demand since the first line was completely occupied. The construction for third injectable line (dry powder and lypo liquid) has also started and expected to be completed by December 2017. The injectable first line has also been fully automated and the company has also constructed additional packing lines for the same. The company has been working on many molecules including, Para IVs, FTFs and 505 (b)(2). It is working 3 FTFs for its customers (contract manufacturing) to be launched during 2022. The company is consciously making efforts to reduce its dependence on one major customer and has not signed up with it for any of these 22 ANDAs filed by the company. During FY16, company derived Rs.78.89 crore from product development charges, out of which ~Rs.10 crore was from API sales while the remaining was
The company is planning to file its first FTF in its own name in the current year. The company has also filed seven molecules for the EU markets and planning to increase it to 14 in a year or two. The approval time lines for EU are shorter compared to US. On account of its IP strength, there was settlement of litigation for 2 ANDAs filed in the US markets. The company only takes head on with innovator if it has IP strength and believes there is non-infringement of the patent. The IP strength of the company has also been appreciated by few of the MNC innovator companies. The company is also entering for direct marketing of its formulation products in some of the markets like Austria, Germany and UK through its subsidiary Koanaa Healthcare GmBH.
For Gemcitabine, the company has also got approval from EU for injectable. Although, the revenue contribution from this is not expected to be huge, but company wanted to get experience of manufacturing formulations for commercial use (instead of exhibit batches it is currently supplying). The company has received approval for the launch of gGleevec (Imatinib) in European markets in December, 2016. However, the company was a bit late in tying up with the marketing partner for the same. The company is pretty sure of getting approval for the same in US markets. The company has calculated approach towards intellectual property (IP) and patents and follows a calculated approach towards it where all the departments like R&D, manufacturing and IP decide upon the selection of the product for R&D, filing and manufacturing. The major revenues from formulation division will only start from Q4FY17 onwards.
Many Indian generic companies like Torrent, Alembic, Zydus etc have announced entry into oncology space but with more than 10 years of experience, earlier entrants like Intas, Dr. Reddy’s and Shilpa will have advantage over them. Some of these companies don’t even have oncology API or formulation manufacturing plants. A company like Shilpa which is backward integrated into APIs and which files dossiers on its own will have an edge over them. Many of these new entrants may even be Shilpa’s customers for formulation as well as APIs.
Japanese CRAMS
The company has started manufacturing exhibit batches of Tranxemic Acid for its partner currently and commercial supplies will commence from June, 2017 onwards. These division has a revenue expectation of Rs.50 – 60 crore with higher margins than CRAMS for ICE. The company is also in talks with other Japanese companies for other opportunities for CRAMS as well and approval from PMDA, Japan has further enhanced company’s prospects of attracting new customers.
Antiretroviral (ARV) Drugs
The company is not planning to pursue it on account of capacity constraints and has surrendered the license to Gilead.
Raichem Medicare (ICE JV)
The ICE CRAMS has seen consistent growth in the revenue contribution over the past few years. The company has been supplying raw ursodeoxycholic acid (UDCA) from Raichem’s plant and has been successfully audited by ICE’s consultant. The ramp up of supplies from the JV will happen FY18 onwards. The manufacturing of UDCA will be gradually phased out of Shilpa’s API facility only over the next two-three years on account of huge shortage of the drug. ICE has access to the key raw material (ox-bile) for manufacturing of UDCA and thus has higher market share. The new facility will have double capacity which is there in the API plant of Shilpa. The company also has long term plans for manufacturing of formulations and also other patented molecules of ICE. The approval for this facility for PMDA, Japan will take at least 2 years. The vacant capacity in Shilpa’s API plant post shifting to Raichem Medicare will be filled by non oncology molecules.
Investment in subsidiary companies/JVs
Philosophy behind investment: The main reason for investment is getting into niche areas which have potential for the future. Shilpa needs to mark its presence like Biosimilars or Nano Technlogy, and /or extend current capabilities around different delivery mechanisms such as Oral Disintegrable. The company makes investments considering at least 3 – 5 years of gestation period. Apart from technology and people running the companies, we also consider future potential and economics involved. The investments in such areas is not much initially but future potential can be huge.
Navya Biologicals: Biosimilars have huge potential in the future. Out of the top 100 pharmaceutical molecules in revenue terms, 45 are biological. Furthermore, out of the 10 new NCE molecules being approved by regulatory authorities, 5 – 6 are biological. We only thought about entering the segment post clear guidelines from regulatory authorities like USFDA and EU. If we entered on our own, it would have taken us at least 5 – 7 years to progress. By acquiring Navya, we saved time as they have been working in the Biosimilars for the past 7 years. They have been working for Indian and unregulated RoW markets. They have a good team but were lacking financial support as well as experience. With our investments and guidance, we can take the company to next level and even work for regulated markets like US and EU in few years. From 3rd year onwards post acquisition, we might see some revenues coming in from these subsidiaries. We are setting up a new facility for manufacturing of Biosimilars for Navya with total cost of Rs.150 crore at Hubli, Karnataka. Shilpa had offered the promoters cash for buying out the company but they wanted Shilpa’s equity. The have an agreement to stay with the company for next five years. Five years down the line, biosimilars might be the second biggest revenue stream for the company after oncology formulations and APIs.
INM Technologies: Nano technology has huge potential in the future. The company is working on both pharmaceutical and engineering projects. Nano technology can have good demand in the future especially in dental field. Many of the pharmaceutical companies talk about ophthalmology and dermatology space but none talk about dental. The company is working on 12 products out of which 2 – 3 are at advance stages of being commercialized. The filings will commence for domestic markets in the near term and gradually progress to regulated markets. Nano technology can help Shilpa in administrating few of its molecules to be administered through this technology through 505 (b) (2) route. The company is planning to set up a new plant with total cost of Rs.50 crore.
Shilpa Therapeutics (formerly Nu Therapeutics): The company has already started working for domestic markets and will enter regulated markets in the near to medium term. The company will start generating meaningful revenues 2 -3 years down the line.
Maia Pharmaceuticals and Makindus Inc, USA: Investing in both these companies have helped us in getting know how of the US markets, dealing with USFDA etc. For Maia, the second round of funding was at four times higher multiples compared to our initial investment. They are at advance stages of filing one to two niche ANDAs for molecules and might even get substantial license fee by end of the year.
Makindus is struggling to get next round of funding of USD 20 – 30 million for phase III trials. We did not have such large funds to support them.
Loba Feincheme GmbH: We recently hired a senior person of pharmaceutical industry, Dr. Walter Erber, to look after the operations of the company. Earlier, a senior employee from Shilpa was managing the operations of the company. He used to visit the facility for few days in a month. With the hiring of a full time person, the operations of the company are expected to be more streamlined. Even Mr. Bhutada is visiting the company regularly to motivate the employees. The company turned profitable last year and expected to grow in the medium term. Shilpa might even use its facility for manufacturing of formulations as well its warehouses. The experience of the company will help us in direct marketing Shilpa’s molecules in Austrian markets.
Koanaa Healthcare GmbH: We have identified Austria, Germany and UK to test waters for our own front end for formulation products. Out of these three countries. Austria is a small market, UK is medium sized while Germany is pretty large. We have hired four senior professionals from the pharmaceutical industry to look after the marketing arrangements.
Other points discussed
Capex and funding plans: The company is planning to spend Rs.450 crore of capex over the next two years. Out of these, Rs.150 crore will be for setting up third dry powder injectable line in formulation facility, Rs.150 crore will be for setting up of plant for Navya Biologicals, Rs.50 crore will be for setting up facility for INM Technologies, Rs.50 crore for API plant and Rs.50 crore for setting up centralized R&D centre at Bangalore or Jadcherla. The funding of the same will be through debt of Rs.150 – 200 crore, cash of Rs.70 crore and remaining through internal accruals. The company was earlier planning to raise equity for the same, however, dropped the plan later. However, now it can raise some debt and through its own internal accruals go ahead with its plans. The company has always believed in not raising huge debt which can lead to its downfall and hence diluted equity to raise funds.
Next generation joining the business: The two sons of Mr. Bhutada have recently joined as trainees in the company. They will undergo training for next two years in various departments and work with even lower level employees. The intention is to make them aware of their responsibilities. The promoters don’t have any business interest outside the company and are fully dedicated in growing the company.
Disclosure: Forms more than 10% of my pf. No transactions in last 30 days.