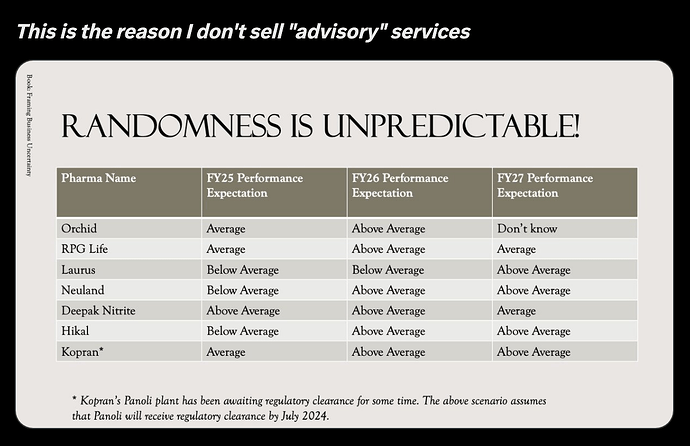
RPG Lifesciences -
Q4 FY 25 results and concall highlights -
Q4 outcomes -
Revenues - 143 vs 127 cr, up 13 pc
EBITDA - 30 vs 23 cr, up 36 pc ( margins @ 21.4 vs 17.6 pc )
PAT ( adjusted for one off gains ) - 18.5 vs 13.2 cr, up 40 pc YoY
FY 25 outcomes -
Revenues - 653 vs 582 cr, up 12 pc
EBITDA - 172 vs 135 cr, up 27 pc ( margins @ 26.4 vs 23.3 pc )
PAT ( adjusted for one off gains ) - 112 vs 88 cr, up 27 pc
EBITDA margins have seen continuous expansion for past 6 years
Company is debt free
FY 25 segmental performance -
Domestic formulations - 425 vs 386 cr, up 10 pc
International formulations - 132 vs 106 cr, up 24 pc
APIs - 90 vs 85 cr, up 6 pc
Company’s manufacturing footprint -
F1 unit @ Ankleshwar caters to domestic and emerging markets, has approvals from WHO, various African countries
F2 unit @ Ankleshwar caters to regulated markets, has approvals from EU GMP, WHO, various African countries
API unit @ Navi Mumbai, has approvals from PMDA ( Japan ), WHO, TGA ( Australia )
Popular brands from company’s stable include -
Naprosyn - Painkiller
Azoran - Immunosupressant
Lomotil - used to treat diarrhoea
Immunotac - Immunosuppressant
Arpimune - Immunosupressant
Mofetyl - Immunosupressant
NuGliptin - Cardiovascular
SacuNew - Cardiovascular
HerMab - Trastuzumab - Onco Drug
AdluMab - Adalimumab - Onco Drug
IvzuMab - Bevacizumab - Onco Drug
In FY 27, new API and International formulations plant will be operational with new product launches. That should lead of accelerated growth in these business segments
Cash on books now @ 266 cr ( post receipt of proceeds from sale of surplus land holdings )
Naprosyn and its variants clocked sales of 76 cr, Immunosuppressants clocked sales of 79 cr for full FY 25
Company’s newer businesses like - Nephrology, Cardiology, Monoclonal anti bodies and Oncology are growing in healthy double digits
Cardiology now contributes to 20 pc of company’s sales
Receivables in Q4 are slightly elevated due higher sales in the international business
As Monoclonal Anti Bodies, Cardio portfolio grow bigger, margins should improve gradually. However, the possibility of some of company’s brands coming under DPCO may exert negative pressure on margins
MR productivity @ 6.3 lakh. MR productivity in speciality divisions is > 13 lakh / MR
Domestic formulations growth for FY 25 by volume @ 7.3 pc, by price @ 2.3 pc, by new product introductions @ 1.1 pc
Price hikes this year are likely to be higher in FY 26 vs 25. Aprox 30 pc of company’s business is under DPCO. The price hikes allowed in the DPCO part of the business in FY 25 was < 1 pc. Also, company faced some pricing pressures in the non DPCO business
Even the new launches in FY 26 should be far higher vs FY 25
The Industry’s volume growth in FY 25 was 1.1 pc vs 7.3 pc for the company ( no mean feat - imo )
If the company is able to maintain its volume growth, bigger price hikes + newer product introductions next year may propel company’s topline growth into mid teens
Company has invested a lot of money in upgrading and modernising its API plant. There are 12 molecules in the company’s R&D pipeline. Company hopes its API business should start to see improved growth rates wef FY 27
Company is also planning to add multiple new formulations to its international formulations business by early FY 27. This should help them keep the international growth momentum intact
API segment’s growth in H1 was in double digits. Then there was an unfortunate fire incident in one of their three API manufacturing blocks. This led to a slower full yr growth in their API business. This should start to reverse wef H2 FY 26
Company lost sales to the tune of 8-10 cr in Q4 due to the a/m fire incident
Disc: holding, biased, inclined to add more, not SEBI registered, not a buy/sell recommendation