granules management has guided for 20% growth both in topline and bottomline for fy16.so far metformin is their only usfda approved drug try the orange book.lot of hard work is needed by mr krishna prasad to get into the big league only time will tell
ibuprofen,paracetamol, metformin,methocarbamol and guaifensin i feel can no longer help granules in margin expansion,also volume growth in these molecules could have peaked these at the most could be steady cash generating molecules provided prices dont drop further.auctus molecules are the jokers in the pack how fast the anda are filed and how many are approved and how many final dosages are made will determine the future of the business.crams with ajinomoto is a fy18 story.my personal opinion
Granules share down by approx 4-5% today due to it being in GDUFA Facility Arrears List.
Complete List below
Can anyone help understand what could be the implications of this notice from USFDA?
My GUESS is that this can be easily resolved by paying the outstanding amount.
Well, it can be paid easily, but the larger question is why was it not paid so far? What internal checks and balances are in place to avoid such a scenario in the first place? This should be a simple but fairly important step to ensure such arrears are paid off?
Somehow, I get a sense that management is not upto the mark?
Disclosure: I do not hold Granules. Somehow, not comfortable with management and there are so many pharma companies making APIs and what distinguishes Granules from others? Migrating to high margin products may not be easy with lot other pharma companies too are trying to do the same. So, I think management is what differentiates one company from another.
Let us wait for the clarification from management instead of drawing conclusion that it is corporate governance issue . Because the Comment section mentioned that
“If your records indicate that a facility fee was previously submitted, please contact AskGDUFA@fda.hhs.gov and provide the following information: Facility name, DUNS, FEI number, User Fee Payment I.D. Number (PIN), and the payment method, date and amount. Additionally, if you feel that your facility is not subject to GDUFA fees, please contact AskGDUFA@fda.hhs.gov and provide a brief explanation for your reasoning. The agency will review your explanation and respond to it promptly. We encourage you to seek verification from your business partners that all applicable fee(s) have been received in full by the Agency in order to minimize the risk that a receipt of an application will be jeopardized. GDUFA Facility Arrears List (07/07/2015) G”. Also I noticed that the report was published on 7th July
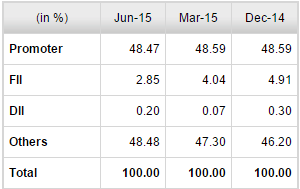
Morgan Stanley sold around 50% of their holdings (5 million out of 10.2 million shares) yesterday. They purchased 10.2 million shares very recently and any reasons why they are offloading it so fast ?
@ Ashish - Granules’ name does not appear in the pdf link sent by you. Am I missing something?
Today, they have updated the list (the pdf link) and removed Granules and a few others.
Yes , At 8:20 PM US Time (9th July15)- 5-55 AM Indian Time (10th July15, the list is found updated and Granules along with few other cos have been removed from the list which indicates the management is proactive to this kind of issue . Refer the same link now for confirmation.
Granules clarified today they already made payment before due date to GDUFA
Still, isn’t Morgan Stanley pulling out, a bad sign ?
I wrote to their IR yesterday night and the response was available by 10AM today.
===========================================================================
We wish to inform you that Granules has paid all the fees before the due
date. We have talked to US FDA on this subject. US FDA after
verification, removed
our name from the arrears list. Today morning, we have sent the
following message to the stock exchanges.
We refer to the GDUFA Facility Arrears list dated 7th
July 2015 published on the U.S. Food and Drug Administration website,
We wish to inform the stakeholders of the Company that all annual
facility fees as required under GDUFA,
was paid by the Company before due date.
We have taken up the matter with the U.S. Food
and Drug Administration and after verification; the U.S. Food and Drug
Administration removed the name of Granules India Limited from arrears
list.
There are always something to worry about…but it’s better to be calm till you know any adverse news with certainty.
By the way does MS has such a great track record that we should worry about their buy/sell?
us fda says NSAID can up the risk of heart disease and stroke especially in patients with heart disease, diabetes, hypertension.plans to issue warning on otc products.NSAID are ibuprofen and paracetamol,TOI 11/7/2015.will docs and patients become cautious before popping painkillers? and will this affect raw material suppliers?
NSAIDs are known to cause vasoconstiction when used for long periods of time. Kidneys and heart are most commonly affected. Paracetamol is relatively safe even after long term use. However, it’'s analgesic potency is less compared to the likes of diclofenac, ibuprofen etc. Most NSAIDS are available only on presription except IBUPROFEN and PARACETAMOL. While NSAIDs are prescribed for short term only, there is always a chance of long term abuse. While this warning keeps coming on and off from various authorities, NSAIDs remain the largest consumed drugs today. Such a warning is a welcome measure to prevent people popping these pills on continuous basis. This will have negligable impact on sales of NSAIDS as such.
Company looking to move up the value chain with the help of acquisitions
Having built a global scale and the capabilities in pharmaceutical ingredient(API) manufacturing front, Hyderabad-based Granules India Limited is now focusing on R&D and formulations and the new acquisitions are likely to reflect this new thrust in its business strategy.
“We may look at acquiring companies having high technology in formulations manufacturing or with a sizable formulations portfolio,” C Krishna Prasad, chairman and managing director of Granules told Business Standard. The company would like to make 1-2 acquisitions preferably in the US and in India if it could find a right company, according to him.
Management thinks that the present API portfolio will help take the revenues to a size of Rs 2,500 crore going forward. The company would bring a set of another 10-20 high-margin APIs apart from a couple of acquisitions to go beyond the present growth potential.
Prasad said the company would target future growth strictly in alignment with its ongoing endeavor to improve profitability in business. "We are not taking unnecessary risk for the sake of growth. We don’t believe in growth at any cost,
I am able to visualise the foll
- margin enhancement due to shift to formulations
- PE enhancement now that both invesco and ridge are out and QIP is on
- FY 2017 eps of about 10 and a pe of about 30
The key question is why do they come out with sound bites close to QIP? do they need to talk up the prices ? Secondly, why can’t they build their own R&D pipeline? This is not a rocket science neither they lack internally generated funds. The R&D purchase won’t come cheap anyway. It looks like all their M&A was to buy growth.
Shareholding Pattern For June 30, 2015