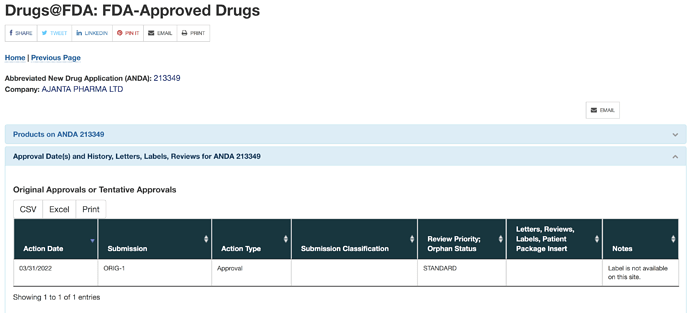
On 31st March 2022, Ajanta got ANDA approval for gBystolic. In the last concall, management had said that new approvals in US will come after FDA inspects their facilities. Given that they have got a new approval it might imply they got inspected by FDA and also cleared it.
Note: This inference is a speculation on my part. Their last ANDA approval (before gBystolic) was in June 2021.
Disclosure: Invested (position size here, bought few shares in last 30-days)