As per the bloomberg data, torrent’s market share in Abilify has dropped slightly to 9% in December from around 10% in November. this can be attributed to entry of Aurobindo, which has managed to grab mkt share of around 2%.
we can get more details on Abilify pricing in Alembic pharma’s conf call. alembic’s results are on 21st, conf call should be on 21st or 22nd.
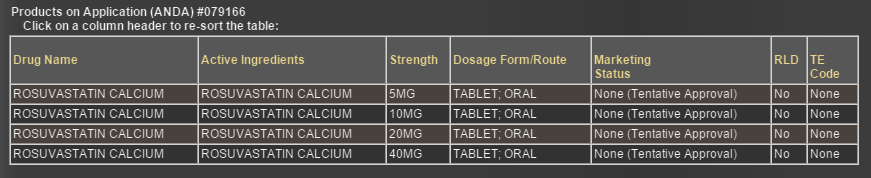
Other 9 ANDA files who have received tentative approval are -
AUROBINDO PHARMA, GLENMARK GENERICS, MYLAN PHARMA, PAR PHARM, SANDOZ, SUN PHARMA GLOBAL, TEVA PHARMS, WATSON LABS, APOTEX CORP
Torrent has tentative approval for only 1 dosage strength (as captured by @lustkills in earlier post) -5mg unlike the other 9 who have TA for all 4 dosage strength as captured below.
TEVA
This probable discrepancy needs to be checked with mgmt in concall.
(Only other reason could be FDA may give TA at a later date for remaining dosages if applied, which IMHO seems unlikely)
Hello @dreddevil27
In simplistic terms:
API - semi-finished goods, not applicable for marketing.
FD - Marketable formulation which requires additional data such as bio-equivalence proof studies, safety/quality tests, etc that has to be submitted with ANDA application.
Please check VP Pharma Sector FAQ for better understanding.
I’m going a little bit out of topic here, so pardon me for this.
I have tried a number of times to understand Pharma sector but it is beyond me. My basic questions or variables to be tracked are:
- Need to track the market size of a product, the competitors in that field, exclusivity period, how the company is placed in capitalising the opportunity.
- Many technical and functional terms to understand.
- How to gauge which field of medicine is lucrative? Like Cancer related, Skin, Diabetics, heart ailments and a number of different fields.
- What ANDAs and DMFs the company is filing and the corresponding market size, if approved?
- What future products exist in the pipeline and again the competitive landscape, pricing power, exclusivity period, off patent price drops etc.
- Pray that there are no FDA warnings/alerts to top this (Of course, the FDA inspections are good for the long term health of the sector though)
- What goes into manufacturing these APIs, Formulations etc. and how the prices of these raw materials change? Basically, they are chemicals only, right? Organic chemistry?
So, we need to keep eyes and ears open to the latest happenings in the Pharma sector and be ahead of the curve in buying and selling.
I only understand that
- The market size is too big and Indian companies have mastered the art of manufacturing APIs, Formulations, marketing.
- It is outside of my circle of competence and so keep out of it.
I’m still studying, and hope to invest if I find enough conviction to do so, until then, I will keep reading all the Pharma related threads closely and thank the members contributing to the knowledge sharing.
richdreamz,
First of all try to figure out where the company’s main revenues come from. Is it US, India, or other geographies. Or a mix of the above. US remains the most lucrative market for India pharma companies.
Next if u zero in on companies focussed on US then try to look at the track record of the company as to how it has used whatever opportunities it has had in the past. Also look at any past problems with USFDA. And most imp is when was the last USFDA inspection done. If it is within last 6-12 months one can be relaxed for next couple of years. (although this is not sacrosanct)
In companies focussed on US markets one needs to have a brief idea about its pipeline. Nowadays most info is available in research reports or on concalls.
And one has to understand that after all the study and diligence the fate of the company’s prospects in a particular molecule rest on the type of competition that turns up at the time of launch of the molecule. And that often is not known till the date of launch.
Many a times companies facing the USFDA heat often offer very attractive entry points due to a problem that is temporary in nature and might get resolved in a year or two.(sometimes it takes longer as in case of ipca but stock price is holding steady) Currently cadila is facing problems and could be watched. The companies facing USFDA problems face following sequence of events.
first there is 483 letter which mentions irregularities observed at the location. Most companies are allowed to export products from these facilities. But if USFDA is not satisfied with the measures taken to resolve the problem it can slap an import alert from that facility.
Both letter 483 and import alert are causes for stock prices to tank majorly.
Often it is easy by looking at the price pattern at which level the worst news are factored in. e.g in case of ipca it was closer to 600 and sun pharma it was closer to 700. (stocks may go still lower if problems persist but thats a chance one has to live with) Dr Reddys seems to be getting support around 2900-3000. I think the ideal thing to do with these situations once one is convinced about merits in buying a particular company is to buy over a period of time. Stagger the purchases over 6-12 months especially on days following bad news.
We have a lot of models to learn from to get on top of the companies facing USFDA music and the sequel of events.
Its not necessary that if Torrent doesnt have tentative approval for other dosage forms, it wont get approval for the same. Many a times, a company who doesnt have any tentative approval, also get final approval from USFDA for the molecule at the time of patent/exclusivity expiry.
Article on how drug makers try to make up for loss due to " Patent expiry "
January 7, 2016 12:00 pm JST
Intellectual property strategy
Drugmakers seek ways to deal with the ‘patent cliff’
KENJI ASADA, Nikkei staff writer

A technician works at Fukui Warp Knitting’s textile factory. The company is collaborating with Teijin to develop pharmaceutical products using textile technology.
TOKYO – Despite knowing well in advance, a company loses 40% of its total revenue overnight and there is little or no hope of recovery. In the absence of a high-profile scandal, companies in most industries would consider themselves extremely unlucky to be facing a situation like this. But the problem is far from rare in the pharmaceutical industry.
The culprit is substance patents, which are where much of the value in pharmaceuticals lies. Once the patent expires, the manufacturer can suffer a huge drop in sales as it falls off the proverbial patent cliff. Consequently, drugmakers are looking at a variety of ways of stepping back from the edge of the cliff, or at least not landing so hard when they fall.
A tiny sensor on a 1-sq.-mm silicon chip – so small it flies away if blown on – could help resurrect one of Otsuka Pharmaceutical’s key products.

Abilify tablets containing sensors developed by Otsuka Pharmaceutical
Partnering with U.S. startup Proteus Digital Health, Otsuka has embedded a sensor in its antipsychotic drug Abilify to enable health care professionals to determine whether patients are taking the drug in accordance with doctors’ instructions. The company applied for new drug recognition from the U.S. Food and Drug Administration in September.
The active ingredients are the same as in conventional Abilify, but the medicine qualifies as a new drug because of the new function. If approved, the company’s intellectual property will be protected under a data exclusivity period of eight years. If any other company were to develop a generic equivalent with a sensor similarly embedded, Otsuka could sue.
The patent covering Abilify as a drug expired in the U.S. in April. Until then, other companies could not turn out drugs with the same active ingredients, but once the patent expired, generic drugs priced at just one-third of the cost of Abilify quickly hit the market all over the world.
CONFIRMING PRESCRIPTION ADHERENCE
In the July-September period of 2015, revenue generated by Abilify in the U.S., the main market, was less than one-tenth of the same period of the previous year. Global Abilify sales in fiscal 2014 totaled more than 500 billion yen ($4.14 billion), accounting for over 40% of group sales for Otsuka Pharmaceutical’s parent company, Otsuka Holdings. Facing such a huge loss of total companywide sales, Otsuka decided to act and hit on the idea of embedding a microsensor.
Otsuka Pharmaceutical’s Abilify antipsychotic drug
The microsensor’s silicon substrate has copper and magnesium electrodes. When the drug is ingested, the sensor reacts with stomach acids and a current flows between the electrodes, causing the chip to emit radio waves. In response to the radio waves from the chip, a patch attached to the patient’s abdomen records that the medicine was taken. The chip is eventually discharged from the body.
Reportedly, 60% of schizophrenia patients do not take their medication as prescribed. Routinely skipping the drug leaves the patient likely to relapse, and in many cases the symptoms worsen.
Making it possible for the attending doctor to monitor dosing history could be a game-changer in providing effective treatment. “This can be applied to treatments for other mental illnesses,” said an official at Otsuka Pharmaceutical’s drug business planning division.
INIMITABLE TECHNIQUE
Other drugmakers have similarly used technology from nonpharmaceutical fields to avoid the patent cliff.
One example is Teijin, the producer of Feburic, a drug for treating hyperuricemia, which can cause gout. The drug boasts sales of nearly 70 billion yen a year worldwide, including consignment sales. However, patents covering it will expire in most countries between 2018 and 2020. Having determined that it would be too difficult, the company abandoned the idea of extending the life of the drug and instead turned its attention to textile technology.
Teijin developed and now produces a “body-absorbent fiber.” This fiber is processed into a cloth and is combined with a hemostatic agent that includes a component for regenerating tissue to treat external wounds. Progress is also being made in processing the cloth into a patch that can be used to regenerate tissue in patients suffering from heart conditions. Teijin is cultivating these products to replace Feburic as its biggest earners following the expiration of its patents.
An official from Teijin’s new business promotion headquarters explained the benefit of utilizing textile technology by saying, “It differs significantly from drugs that can be created by anyone once they understand the structure.” Production technology is being developed jointly with textile company Fukui Warp Knitting, allowing Teijin access to valuable expertise and production equipment.
This represents something of a return to the company’s roots, having originally moved into pharmaceuticals from textiles. More importantly, this is an approach that Teijin believes other companies cannot easily replicate.
SOFT LANDING
Rather than add new features to existing drugs or develop entirely new ones, some drugmakers have pursued more of a “parachute” approach. One such company is Shionogi, which has come up with an astute way of mitigating the impact of the 2016 expiration of patents covering Crestor, a drug for the treatment of elevated cholesterol and triglyceride levels.
Shionogi has negotiated a deal with British pharmaceutical giant AstraZeneca, which holds the sales rights for Crestor outside Japan, whereby royalties from Crestor sales are significantly reduced in return for an extension of the contract.
Even after the patent expires, Shionogi will receive regular income from AstraZeneca’s sales of Crestor, albeit at a reduced level. This arrangement buys time for the company to develop new drugs, including treatments for HIV and influenza.
There are some drugs for which sales do not slump significantly even after the patent cliff. One is Takeda Pharmaceutical’s prostate cancer treatment agent, Leuplin.
The patent for Leuplin expired in the latter half of the 1990s, but global sales in fiscal 2014 were 124 billion yen, down just 2% from the prior fiscal year and virtually unchanged from the most recent peak of 127.5 billion yen in fiscal 2006. When compared with cases such as Takeda’s diabetes treatment drug Actos, for which post-patent-expiry sales fell by around 90%, the difference is stark.
The secret is the microcapsule that contains the active ingredients of Leuplin. The capsule is designed so that after it enters the body, the components seep out over an extended period of time. The drug remains effective for up to six months with a single dose, eliminating the need for monthly injections.

A sign at a pharmacy in Tokyo recommending the use of generic drugs
The microcapsule is made of poly(lactic-co-glycolic acid), or PLGA. Primarily used in surgical sutures, this substance gradually breaks down inside the body. Using proprietary technology, Takeda created capsules from this material and filled them with the drug’s active ingredients.
This technique is one that other companies have so far been unable to replicate. Moreover, the company has built up a solid reputation among doctors after changes to the drug lengthened the period for which it remains effective.
The war against the patent cliff is synonymous with the war against generic drugs. If medicines earn the trust of health care professionals, patients don’t immediately switch to generics even after a patent expires, meaning earnings don’t erode. Such is one of the “soft landing” strategies utilized by pharmaceutical companies.
OVERSEAS ACQUISITIONS
When faced with the prospect of falling off the patent cliff, the typical response of major drugmakers in Europe and the U.S. is very different from that of their Japanese counterparts. Many invest significant capital to try to secure the next big new drug by buying up other companies or investing large sums into development in specific fields.

The production line for Takeda Pharmaceutical’s Leuplin in Yamaguchi Prefecture
Since 2000, U.S. drugmaker Pfizer has epitomized this approach by making repeated acquisitions, including the purchases of U.S. pharmaceutical companies Pharmacia and Wyeth. As the expiration of patents covering its cholesterol-lowering medication Lipitor – which boasted sales in excess of $8 billion – approached in 2012, it steadily undertook this countermeasure. Last year it agreed to merge with Ireland’s Allergan and is rapidly expanding its roster of drugs in development.
Faced with the expiration of patents on its antihypertensive agent Diovan in 2014, Switzerland’s Novartis took on a new drug candidate in the cancer field from British drugmaker GlaxoSmithKline and is moving forward with research and development by investing in excess of $8 billion a year.
Drugmakers seek ways to deal with the 'patent cliff' - Nikkei Asia)
Another launch from Torrent in domestic market http://www.business-standard.com/article/companies/torrent-launches-world-s-second-biosimilar-of-generic-auto-immune-drug-116011100615_1.html#.VpPCmpNUDRQ.twitter
Reliance Life Sciences gets US FDA nod for Navi Mumbai plant
[Since Torrent has tie up with Reliance life sciences, news posted here]
Conceded new ground yday and the trouble continued today…now below 1350…
Painful to watch this but gritting my teeth and accumulating some small quantity since that is all I can do now. Anyone else, accumulating it? The market continues to find new ways to decimate midcap pharma in general. Hope the election year stunt of FDA doesn’t rear its head here.
Would start SIP if it breaks 1300 and then 1270
@All pharma experts,
The below article is worrying. Is it time to reassess pharma sector allocation?
http://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/a-strict-fda-competition-may-slow-pharma-growth-export-may-halve-to-8-per-cent-by-2020-study/articleshow/50632089.cms
The growth rates may or may not decline. However if enough people get bearish, PE derating is surely on the cards. Case in point - in the last para, author says that India to US exports rose from $3.44 bln to $3.76 bln. in 2014 and we are in 2016.
As Howard Marks says, the sentiment swings from one extreme to another.
announcement by torrent pharma. interim dividend to be considered.
Torrent Pharmaceuticals Ltd has informed BSE that a meeting of the Board of Directors of the Company will be held on February 04, 2016, inter alia, to consider and approve the Audited Financial Results on Standalone basis and Unaudited Financial Results (with limited review) on Consolidated basis of the Company for the quarter and nine months ended on December 31, 2015 (Q3) and would also consider payment of Interim Dividend for the year 2015-16.
The management can do three things with the windfalls (sort of) they made this year-
- Distribute-via dividends
- Repay Debt
- Build up cash for organic and inorganic growth
Management has already stated its inclination towards 3rd option and has categorically rejected the second one.
Let us see how much do they allocate towards the First option.
Hoping for the Best.
Torrent, Dr Reddy’s, Aurobindo, Cipla, Lupin in race to buy Sagent
By Vikas Dandekar, ET Bureau | 25 Jan, 2016, 03.32AM ISTPost a Comment
MUMBAI: At least five Indian drug companies — Torrent Pharmaceuticals, Dr Reddy’s Laboratories, Aurobindo Pharma, Cipla and Lupin — are in initial discussions to bid for Sagent Pharmaceuticals, a Nasdaq-listed specialty injectables maker, sources familiar with the talks said.
The discussions started about a month ago, they told ET. Sun Pharmaceutical Industries was also interested and had even initiated the due diligence process, but then backed out, mainly over valuation, the sources said.
Sagent has mandated New Yorkbased investment banker Perella Weinberg Partners to scout for potential opportunities as part of its strategic alternatives.
Valuation is expected to be upwards of $500 million (Rs 3,400 crore), for which large generic drug makers and financial investors like global private equity funds may also be interested.

Torrent, Dr Reddy’s, Aurobindo, Cipla, Lupin in race to buy Sagent Sagent didn’t respond to an email ET sent last week seeking comment. Cipla said it is constantly in discussions with multiple parties on potential collaboration opportunities in line with its aspiration to drive access and ensure availability of high quality, affordable medicines, but refused to comment on any specific talks. The other Indian firms that the sources said were in talks didn’t respond to request for comment.
Sagent presents an ideal opportunity for companies that have an injectables manufacturing platform with focus on hospital-based medicines, industry watchers said. “Sagent has a tailor-made injectable sales and distribution outfit that could suit Indian companies. But the injectable business is getting highly competitive so the margins are getting squeezed. This is the right time to exit (for current investors), as money is available and valuations will be better,” a senior executive said.
Besides, with an increasing number of Indian drug makers faced with tough regulatory actions from the US Food and Drug Administration, the executive believed, the appetite for complementary manufacturing sites and products is high among Indian companies.
Sagent’s marketed products include calcium leucovorin, gemcitabine, octreotide, zoledronic acid and oxaliplatin in the anti-cancer drugs segment. Sagent has a set of anti-infective and drugs for critical care like propofol, adenosine and heparin, where it claims leadership positions.
Sagent recently forecast sales of $325-365 million for 2016, representing a compounded annual growth rate of 14% since 2013. It expects adjusted Ebitda of $35-50 million for the year.
The company operates through a network of 46 global partners. This offers the benefits of efficient manufacturing, diversified supplies, specialised facilities, capital efficiency and a broad development platform.
Founded a decade ago, Sagent’s portfolio of 55 products addresses a $2-billion opportunity and it plans a major future scale-up. The company has a pipeline of 98 future products that to gain access in a $13 billion market for injectables, which can further expand to a $37 billion opportunity by 2020.
Last year, top Indian companies saw hectic M&A activity. In the biggest ever overseas acquisition by a pharmaceutical company, Lupin acquired US-based Gavis Pharma in an $880 million deal to shore up its products pipeline. Cipla bought Invagen for $550 million as part of its US market strategy.
For Indian drug makers, the most sought-after market is the US, where as much as 86% of the total dispensed prescriptions are for generic drugs. However, in terms of value, branded prescriptions account for roughly 70% of the total US spending on medicines, which totaled $329 billion in 2013, according IMS Health data.
Hitesh,
Even last year, Torrent pharma did announce interim dividend. So unless, extraordinarily large, it is normal for Torrent to declare interim
http://www.moneycontrol.com/company-facts/torrentpharmaceuticals/dividends/TP06
Last Jan 2015, the company declared interim of Rs 5 per share. Even in Jan 2014, it declared Rs 5 per share as interim. So I expect around 5-6 per share as interim.
Injectibles company does seem interesting for Torrent. MCap as per google finance now is $263Mn which fits the bill for Torrent. But as per news below it is 500mn+
Sagent Pharmaceuticals, Inc. (NASDAQ:SGNT), formerly Sagent Holding Co., is an injectable pharmaceutical company that develops and sources products that the Company sells primarily in the United States. Sagent focuses primarily on generic injectable pharmaceuticals. Sagent offers its customers a range of products across anti-infective, oncolytic and critical care indications in a variety of presentations, including single- and multi-dose vials, pre-filled, ready-to-use syringes, medical devices and premix bags. As of October 31, 2010, Sagent marketed 21 products. The Company has a 50/50 joint venture known as Sagent Strides LLC (Sagent Strides) with a subsidiary of Strides Arcolab Limited (Strides), which is an Indian manufacturer of finished pharmaceutical products.
Found this on nasdaq website
Sagent Pharmaceuticals had a rough 2015, falling 38% because it failed to meet its own expectations. The company started the year with 2015 guidance of net revenue in the range of $325 million to $375 million. In March, founder and CEO Jeffrey Yordon retired and the company’s president, James Hussey, left the company. By the third-quarter conference call in November, the revenue guidance was down to a range of $305 million to $330 million.
Now what: Buying a company solely because you hope it’ll get sold is akin to gambling since it’s hard to know whether other companies might be interested in buying at the current price.
That being said, there’s a lot of consolidation in the generic-drug business where small margins make it advantageous to be larger. Because the acquirer can reduce redundancy, it’s possible that Sagent Pharmaceuticals could be taken out at a higher price than investors are willing to pay for the stand-alone company.
http://www.nasdaq.com/article/why-sagent-pharmaceuticals-inc-jumped-today-cm565444
Shares of Sagent Pharmaceuticals (NASDAQ:SGNT) traded up 2.49% on Monday, hitting $16.48. 159,729 shares of the stock traded hands. Sagent Pharmaceuticals has a 52-week low of $12.78 and a 52-week high of $30.75. The firm has a market cap of $540.33 million and a PE ratio of 20.63.
Otsuka Sues Orchid Pharma to Halt Generic Abilify
By Cristina Violante
Law360, New York (January 26, 2016, 7:41 PM ET) – Otsuka Pharmaceutical Co. Ltd. has hit Indian drug maker Orchid Pharma Ltd. and its subsidiaries with two patent suits in New Jersey federal court over generic versions of the high-selling antipsychotic Abilify.
Otsuka claims in the suits filed Friday and Monday that Orchid has infringed several patents relating to aripiprazole, a prescription treatment marketed as Abilify, which is used to treat schizophrenia, bipolar disorder and Tourette syndrome. Orchid allegedly filed Abbreviated New Drug Applications with the FDA to sell a generic version of the product in…
Results announced:
Consolidated (in crores):
Qtr. : Q3 FY16 v/s Q2 FY16 v/s Q3 FY15
Sales : 1515 v/s 1655 v/s 1156
PAT : 483 v/s 568 v/s 167
Tax : 78 v/s 164 v/s 34 This was alluded to by management in Q2 concall. Q1 tax was 410
Interim dividend of Rs. 20 declared
Thanks @pikrohit @ankitgupta @hitesh2710 and all boarders for the wonderful analysis on visibility.
Which one seems a better bet now: Torrent or Alembic?
Alembic Q4 results will reflect sales of Abilify they did in Q3. Apparently the margins have not taken a big hit. I feel both are undervalued on a 2-3 year forward basis, but Torrent more so, especially on a 2 year forward basis (if and when gAbilify margins take a large hit); though I am not an expert. Would love to hear views of others on this.
Excerpt from press release page 10 (italics are my addition):
Revenues from US operations for YTD FY2015-16 registered growth of 255%.
Current quarter and YTD has continued benefit of high sales of our largest product (read as gAbilify). However, base sales also recorded exceptional growth with support of new launches Esomeprazole and generic Detrol.