What was offensive in my post.?
Can u please post the latest export data for ibuprofen
IOLCP down 30% in 2 days , is this an opportunity to buy ? Those who tracking closely kindly throw some light ? if the correction is because of high valuation and broader market correction or is there something fundamental specific to IOLCP? Heard that BASF has given poor quarter result , can that be reason for such a steep fall?
is it looking good at the current price. definitely. but anything below 150 should be good price to enter.i dont know about the technicals. but BASF plant has not opened up meaning q3 will be as strong as q2. and we are in february and still there is no news on BASF plant opening. se4niors please opine. but its over reliance on ibuprofen alone is a dampener.
@Prasadkumaresan Tanfac benefitted similarly due to Sterlite copper plant closure (Sulphuric acid supply was hit) and Q3 will most probably be very good but market started de-rating it way ahead soon as positive news started coming out about Sterlite copper re-opening. Its currently down about 50% - that’s how quickly things get discounted in these sort of special situations affecting supply. Got to be nimble.
Disc: No holdings in IOLCP or Tanfac
IOL appears to be a different case in point. For the concerned product Ibuprofen, there are only 5-6 manufacturers globally out of which 2-3 major ones are from India. So its an oligopolistic setup, and even if BASF resumes production, prices won’t fall off the cliff because
-
It’s a global oligopoly, producers would have the willingness and ability to control prices in a scenario of global shortage which has been prevailing for the last two years.
-
Isobutyl Benzene (IBB) Prices - IBB is the major raw material for producing Ibuprofen. Vinati Organics and IOL Chemicals (fully backward integrated to produce IBB) control bulk of the global IBB supply. Thus, Ibuprofen prices are dependent on IBB prices which is a tightly controlled market.
The other point to consider is the leeway and business strength that IOL gets due to the current situation, which it can utilize either to significantly pay off debt or prepare for future expansions. They are likely to do a bit of both, thereby strengthening and derisking themselves in the process.
Attached file is their environmental clearance document for expansion. PFR Expansion April 2018.pdf (811.3 KB)
@phreakv6 sir. If my understanding is correct the total requirements of ibuprofen cannot be supplied by IOL and BASF TEXAS PLANT as well. If that plant opens up margins will come down but the supply crunch will be present.
So that should help company post good numbers even after the TEXAS plant opens up. But once BASF plant in Germany opens up then supply demand mismatch will not be there. Hence my consideration is IOL should consider development of other API s which can increase the sales component and reduce the reliance on ibuprofen alone
Connecting the dots (Ibuprofen and IBB)…
Ibuprofen prices last year increased by 20-25% due to Plant closure by BASF which is one of the major manufacturer of Ibuprofen.
BASF plant located at Bishop, Texas has a capacity of 5000 TPA. At the start of first quarter BASF plant shutdown due to technical reason, taking away almost 1/6th of global production capacity off grid. With no capacity addition happening globally, prices tightened due to supply demand mismatch. At the end of FY18, the demand for Ibuprofen was for nearly 40,000 TPA and the global capacity was at roughly 35,000 TPA. IOLCP is a major player in Ibuprofen manufacturing, accounting nearly 30% of global capacity. It has recently de bottlenecked its manufacturing facility and has increased the capacity from 7200 TPA to 10,000 Tons per Annum.
Source: https://capitalmind.in/2019/01/outlier-in-focus-a-quick-view-of-iol-chemicals-and-pharmaceuticals/
BASF Ibuprofen plant shutdown helped IOLCP; visible in good topline and bottomline growth in the last couple quarters (and may continue in Q3Fy19).
Key question to ponder is can this good times sustain?
Iso Butyl Benzene (IBB) is key ingredient for manufacturing of Ibuprofen. Vinati Organics supplies IBB to BASF (source here).
“IBB (Iso Butyl Benzene) volume which was down past three quarters has picked up starting Q3Fy19 and we will see a higher volume ramp up in Q4Fy19 and Q1Fy20 for IBB. One of the larger users of IBB had some breakdown and shutdown for three quarters (name not given; but is obvious) and that shutdown has been rectified and so the plant is starting and our off take of IBB is going to ramp up from Q4Fy19.” ~Vinati Saraf (source here - snippet from interview dated Jan 31, 2019).
Thus, if BASF plant breakdown/shutdown has created Ibuprofen demand supply mismatch, then BASF plant starting again will clear Ibuprofen demand supply mismatch in all likelihood.
Thanks for this important update, Sandeep!
basf plant to start around april 2019. listen in at 4.58 mins
Source : https://www.youtube.com/watch?v=YX7VLfzQNTk
Thanks! this info seems to be more official than the earlier one which indicated BASF restarting the plant in Dec, 2018. Also, this means IOL can continue to reap benefits till end of FY19.
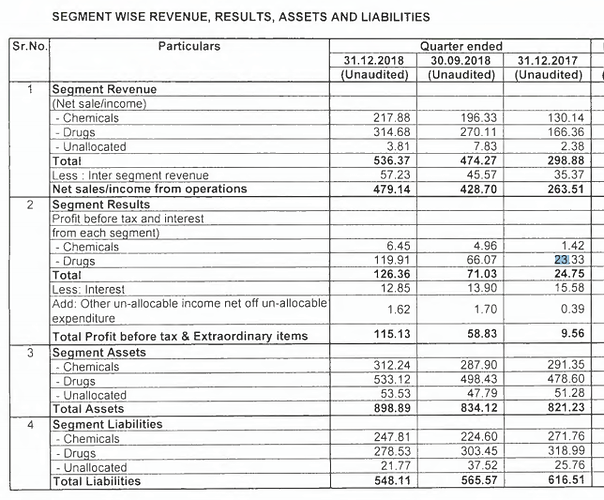
Dec 2018 result is out for IOL Chemicals & Pharma.
EPS is more than anyone would have expected!
EPS is 14.63 vs 1.56 YoY. Prev quarter EPS was 6.84
Sales is 478 vs 262 YoY.
Seeing segment-wise, result looks better because it is not just drugs but Chemical segment also given stellar profit.
Disc: Not invested
The result has been very good, from the notification on BSE they are issuing a warrant at Rs 205, can experienced members give their opinion on this and how we has investors should see this.
Disclaimer : Invested
Dec results have surprised quite positively although not sure how sustainable the profits are. Will be interesting to see how they utilize the funds generated from the super normal profits. I would expect a substantial reduction in debt by March2019.
Promoter subscribing to 2.5 Million warrants at 205 -
- That would provide additional INR 500 M to the company. Not sure the company is in need of funds at this time when they are generating super normal profits already. Their internal accruals should have been sufficient for any debt prepayment / capex plans
- The other reason could be that the management is keen to have a higher stake in the business generating such super normal profits. Subscribing at 205/- (which is broadly the CMP of the company) would probably portray that they continue to be quite bullish on the prospects of the company, although buying from the open market would have sent a better signal IMHO
Disc: Invested
Hi All,
Management interview post Q3FY19 Call, please click here.
Regards,
Yogansh Jeswani
Disclosure: Tracking
Vijay Garg, Jt MD , says, the 50 Cr from pref allotment will be used to create new production of new drugs . That is a positive for me. They will be decreasing the debt as well and that augurs well for IOL Chem too. I feel the management are looking at other Pharma products to commercialize. Keeping my fingure crossed.
I am invested.
Go though this…
The system had failed at the beginning of June and was supposed to go online again in September. The repairs took longer than expected. The reason for the production stop was a technical error: a component that is important for the production process was in need of repair.
BASF has been producing ibuprofen since September 1992 in Bishop, Texas. The location was already affected by production losses in August 2017, the consequences of which were felt in this country during the flu season 2017/18. The reason for this was the hurricane “Harvey”, which led to power failures and as a result to production losses. With a capacity of 5,000 tons per year, the plant is one of the leading producers of ibuprofen worldwide. About one-sixth of global demand comes from BASF, and in 2021 the group plans to commission another plant in Ludwigshafen.
The other five ibuprofen producers of Active Pharmaceutical Ingredient (API) for the world market are currently Hubei Granules-Biocause and Shandong Xinhua of China, Solara and IOLPC of India and SI Group of the USA. The market shares are approximately evenly distributed, which speaks for the utilization of the entire capacity. Each of the six factories produces between 10 and 20 percent of the world market.
English Translation of the above link…Thank you.
Sorry…you only translated second page…
below is first page translation which has MAIN details:
At the end of January, BASF had successfully completed the mechanical commissioning of the plant. In the next step, the devices and connections were cleaned. The pipelines between the individual units alone are 25 kilometers long, plus around 150 units necessary for ibuprofen production. The inspection and maintenance work is correspondingly time-consuming. 350 additional employees are currently busy at the chemical company to get the site back online.
After completing the quality controls, production should start again from next week and be successively increased to commercial regular operation. Orders are according to a spokesman, first the orders from last year are processed. Once the first GMP-compliant drug is produced, delivery begins.
It will take some time before the plant is working under full load again. According to the spokesman, the system will be shut down in May due to maintenance work, and the scheduled shutdown will take another five weeks. After all, in August and September not only the previous capacity should be reached, but additional volumes can be produced. Actually, BASF had already wanted to expand the location a year ago.
Bottomline: BASF TEXAS factory is starting next week as per this…