Trastuzumab review postponed to 03dec. I read it as positive as it gives Biocon time to sort the 483’s & also FDA to review it thoroughly instead of seeking a re-submission. I have no exposure to the name
Yes. But markets are finicky and may react to news. Investors look for a monthly execution rule book but real life is not same. Biocon continues to be one of the best biosimilar players globally.
Fair point -Just my two pennies on technical
We are at whats called extreme levels on MACD. An earlier level was seen on 25th May 2017 & stock thereafter moved higher from 295-439 ish. I see a similar pattern on charts & hence believe that we could head to around 425+ over the next 3-5 months.
No two ways about it-its a great bio similar play -They just need to get their regulatory approvals in place-it is high time they sorted it out considering its a very experienced management
Discl : I have no exposure
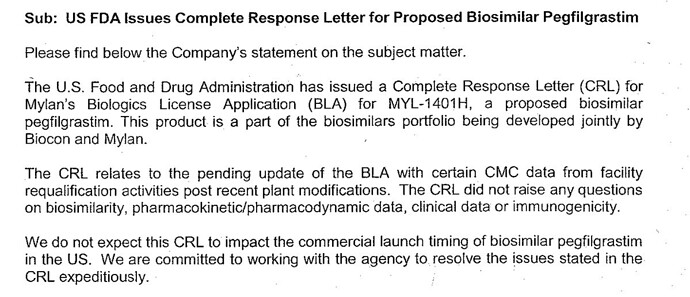
Thanks Monk for sharing the news. I read it as positive as well, if the FDA intended to reject the application for safety/efficacy concerns or other reasons & send a CRL (Complete response letter) they could have, I’m taking this as a huge positive, its only the manufacturing facility that is holding them back and not the product or any of its characteristics. Additionally, the 3 month delay will not affect its first to file status, exclusivity or commercialization timelines (given 2019 patent expiry) even though Amgen is catching up.
Hopefully Biocon will get all its ducks in a row on the manufacturing front between now and December.
Extension of target action date is normal, and has happened for others in the past.
Abaloparatide (Tymlos) from Radius Health got a 3 month PDUFA extension in March 2017 . Extension was given to review additional information submitted by the company.
The drug got approved subsequently in April 2017.
http://investors.radiuspharm.com/releasedetail.cfm?releaseid=1016937
http://investors.radiuspharm.com/releasedetail.cfm?releaseid=1023557
So agree with @rks00 and @monk88888 to take this new development positively.
This is, assuming that the extension of TAD(target action date) is for reviewing additional information of trastuzumab submitted to FDA. If subsequently the TAD for pegfilgrastim is also extended, we will have to presume that these extensions are related to the 483s issued for the drug product facility which is common to both.
I am not so sure it is a positive. The price today includes expectations on positive developments on biosimilar approvals. So anything that delays the biosimilar pipeline is a negative. I am hopeful too that in the long run biocon is able to pull this through. But to term a delay in TAD as a positive sounds more like a buyer’s bias to me. The argument that it could have been worse is not correct as the price has built into it a moderate success case scenario not a worse case scenario. There is still quite some work to be done by business and at present this is really a speculative “investment” for me.
Another note from MS that came late night yesterday summary as follows:
They believe its a better outcome than directly receiving complete response letter. This would provide more time for the company to finish pending cGMP work & FDA to review additional information
The next key milestone to watch is FDA action on 09th Oct-BsUFA date for biosimilar pegfilgrastim.They remain OW
New Steps To Strengthen FDA s Inspection And Oversight Of Drug Manufacturing
"As another key step towards achieving these goals, the FDA s Center for Drug Evaluation and Research (CDER) and the Office of Regulatory Affairs (ORA) are implementing a new, historic concept of operations agreement to more fully integrate the drug review programs with the facility evaluations and inspections for human drugs… This new, team-based approach aligns field and review staff so that we can make closer consideration of all elements that create risk including the drug substance, the drug product, manufacturing processes, and the state of the facilities we regulate… The new model will cover Pre- and Post-Approval Inspections, Surveillance Inspections, and For-Cause inspections at domestic and international drug manufacturing facilities that FDA oversees… We hope that by communicating more quickly with product developers when manufacturing problems are identified, this agreement will help make inspectional issues less likely to cause approval delays or prolong the time it takes to get important products to patients who can benefit from them… "
BIOCON’S Insulins facility in Malaysia received EU GMP Compliance certificate
I am not sure if this has been reported or debated
For me this is an important milestone
USFDA commissioner Scott Gottlieb is involved in the implementation of 21st Century Cures act
"The cures act authorized USD 500 mio over 9 years to help FDA cover the cost of implementing the law-Drug & device companies could see quicker , less expensive paths to approval for new medical products & has highlighted the law’s “breakthrough device” designation and the new oncology centre of excellence which aims to boost anti-cancer initiatives"
I would suggest please read the initiatives by he new FDA commissioner-very positive
Withdrawal letters for Ogivri (trastuzumab) and Fulphila (pegfilgrastim) are available at CHMP site.
http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500234843
http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500234815
EMA was of the opinion that approval could not have been given to both, mainly due to GMP issues at the manufacturing site. They did not mention any issues with the CMC and clinical data submitted.
This bodes well for Biocon, as approval is almost definite on resubmission, if they can take care of GMP issues at the plant.
The withdrawal letter also mention that CAPAs have been implemented and the plant would be ready for reinspection from October 2017.
My only response to you doctor would be to recommend reading a book called “The art of thinking clearly” by Rolf Dobelli. Especially the section on “confirmation bias” starting page 23.
Disc: I hold biocon as a speculative buy
I know I am biased , cant help it though☺
Have read the book u suggested, but left it midway as i felt it was just summarising what Nassim Taleb had written in Fooled by randomness.
Sanofi Files Suit in the U.S. to Defend Its Patent Rights on Lantus® and Lantus® SoloStar®
Paris, France – October 24, 2017 – Sanofi (EURONEXT: SAN and NYSE: SNY) today filed a patent infringement suit against Mylan N.V., Mylan GmbH, Mylan Inc., and Mylan Pharmaceuticals Inc. (collectively, “Mylan”) in the United States District Court for the District of New Jersey. Sanofi alleges infringement of 18 patents in its suit.
The suit was triggered by notifications received from Mylan beginning in mid-September, in which Mylan stated that it had filed a NDA (505(b)(2) New Drug Application) with the FDA for insulin glargine pre-filled pen and vial drug products. Mylan also stated that its NDA included a paragraph IV certification challenging all of the Sanofi patents then listed in the FDA Orange Book for Sanofi’s Lantus® (insulin glargine injection, 100 Units/mL) and Lantus® SoloStar® products.
Q2 bad numbers main reasons & update as stated by management
• Plant modification for regulatory compliance led to production disruption
• Filed Insulin glargine in USFDA via 505(2) pathway
• Best employer for vision, innovative & socially responsible
• Regulatory and tender delays in some emerging markets
• Malaysia facility costs
• Pricing pressure in API small molecules business (Sectoral issue)
• Malaysia facility receive EU GMP clearance for insulin glargine
• Bangalore facility gets GMP for insulin glargine from NRPA, Malaysia, MFDS & South korea.
• Collaboration for insulin tregopil with JDRF for TYPE 1 diabetes
• Branded formulations was good in india and UAE
• CAPA for EMA Europe done from our side and waiting for next steps in re-inspection. MAA withdrawals were part of re-inspection procedure as explained earlier
• Pegfilgrastim CRL was related to plant not any problem with DRUG
• The FDA also extended the target action date for the biosimilar
Trastuzumab application to Dec 3, 2017
to review some of the clarificatory information submitted
to them as a part of the application review process
Disclosure:Invested
Since Mylan seems to be a major culprit in the price fixing conspiracy of US generic companies, what are the repercussions for Biocon as they are entirely dependent on Mylan for marketing their products in the EU & US?
Mylan stock jumped 3% last night. So market seems to have taken it in its stride and decided to move on. Govt and corporate tussle are not unusual. Biocon gets impacted only if Mylan operations are halted. As most of the US generic companies like Teva, Sandoz, Dr Reddy have been named it is unlikely that these companies will halt operations. They may be fined or individuals penalized. Life will go on.
Disc: Invested with 8% of portfolio. My views likely to be biased.
Sold 2/3 of my holdings after big upswing for last 2 days. My fair valuation estimate was 400.
Just hedge against unknown. Continue to believe in long term story. Would be a buyer with 10% + correction.