Thanks Ankit. It’s such a positive development which may potentially ease the overhang on the stock due to 483s…
With all the respect to the board members who have done wonderful work here…just posting my views as to why I believe the stock may not provide decent returns in the near future…
IMHO, 483s is not the only reason why this stock is languishing at 15/16x PE. As per the above discussion, its clear that the Sales for FY17 is likely to be ~ 3000 Cr, so there are no growth expectations. It can very well go thru a long consolidation phase between 550 - 650, just like what is happening with Suven. May provide V good buying opportunity in the coming future. Invite comments…
3000 CR sales without abilify is a latent signal for growth. Although heavy R&D expenses will depress the bottom line (as Alembic adopts the policy of not capitalizing R&D costs unlike other companies such as granules India), PE might be expanding to 25ish level as the expected sales of 3000 cr would be devoid of any windfall gains. Now assuming earnings for fy17 to be similar(or a little less) to fy16, expansion of PE would only happen if price of the stock rises.
Disc: Invested in Alembic and granules
Concall notes
USFDA inspection of formulations plant on 21st-25th March, 4 observations. Last year was zero observation. Submitted responses. No instances of data integrity or management negligence. One plant (API) was not audited since 3 years and 1 was audited within a year.
Only way for sustainable growth is ramping up R&D. 122cr in FY15 to 307 cr in FY16. Ready to spend 450cr+ in FY17. Drop in margins but imp for future. Some companies do 15-20% R&D expense. “R&D expense to sales number is a derived formula. We don’t think that way.”
Focus on Injectibles, derma, onco going fwd
Don’t capitalize on R&D cost as its not good for long term investors.
JV with Obicular- Aleor. Scanned derma space and shortlisted 40 projects to work on. Total USD 5bn market size. 14-15 projects are in advanced stages of development. Launches beyond FY19 because they will require building facilities. JV will need 80-100cr capex in next few years.
What made you go for JV? Partner capability? We don’t have skill set. It will take time 2-3 yrs to develop them on our own. Open to more JVs with partners with complementary capabilities.
11 launches on front end, will be more in FY17
Pranav US business, Shaunak domestic business.
Domestic- Muted. More price curbs of FDP fixed drug pricing. Focus will be on productivity… not much increase in field force. Sikkim will run at full capacity in 16-17
DMF of oncology filed- long term plans? Onco is focus area. Onco Injectibles and onco oral solids plants coming. Many drugs will expire in 6-7 years. Good progress.
Contract manufacturing- Not doing anything. Nothing in future. We do outsourcing for domestic. US is all captive.
Gross margins used to be 60-65% but with abilify its 75-76%. There will be pressure on margin due to increase in R&D as % of sales. Pre-R&D expense, the margins are improving.
ANDA files are lower than internal targets, should see a ramp up in FY17-18. No guidance. Filings in injectibles, derma and onco will increase once facilities are ready.
Projects increased from 50 to lot more….more outsourced R&D projects….expanded Hyderabad R&D. targeting products more aggressively.
Abilify was lower than last quarter because share drop from 8 to 4%. Now share has gone up but there is pricing pressure. Ex-abilify has grown….didnt disclose but said base business was around 110-120cr ex-abilify. including abilify its now 190cr.
Cash of 450cr on balance sheet.
Sharp increase in API…dont expect it to grow a lot. One time opportunity when a customer gets market share and ramps up orders.
Namenda and celecoxib both have small share….competition is intense.
Can you tell why Capitalizing R&D cost is not good for long term investor ? IMO RnD is something with future economic benefits, it should be treated as an asset rather than an expense, it eventually increase the value of the company.Also as the costs wouldn’t be treated as expenses, the profit will be higher and margin will not be affected .
My limited understanding…Hitesh has been asked this question on his thread. Will wait for his explanation
I feel that charging-off the R&D expense to P&L is in principle a correct practice because the money is being spent in that year even though the benefits would accrue in coming years. Its closer to a cash flow idea. It definitely depresses the EPS and thats the reason most companies capitalize on such expenses (and it is allowed under acccounting principles).
When Alembic charges-off R&D to P&L, it shows that they are not too much bothered about the market reactions to EPS numbers. As an investor, it is good if the company is reporting an optically lower EPS because if you adjust for it, the valuations are better for us if we want to buy. As an example (hypothetical numbers), everyone may take the PE to be 25 but the adjusted PE could be just 20 or something, so that is good, Also, understanding financial statements becomes easier for us as they become clutter free.
Totally agree with you Rohit. Although in the final analysis capitalization moves from Balance Sheet to P&L in form of depreciation but directly moving it to P&L ensures that the earnings are not artificially inflated. Assuming that the promoter intent is honest R&D cost capitalization assumes that the projects will necessarily generate earnings whereas expensing them takes that risk out. One another advantage that I think is that expensing R&D gives you immediate tax benefits whereas capitalizing would defer the tax benefit until the expense is depreciated, think of the interest benefit arising (some CA should verify this) . At 450 crores of R&D that would be quite a sum.
As a precaution I generally desist from investing into companies which capitalize R&D.
@Anant thanks alot for the explanation. @nishantkandoi plz note
The difference between 2 replies is an example of first level and higher level thinking. ![]()
Would be interesting to know the interest benefit part Anant is talking about. Can anyone throw some light? @abhishek90 any comments on this?
Hi Rohit,
Anant is basically talking about the tax benefit part where you increase your expenditure and hence your profit before tax declines resulting in lower tax. However, I doubt any listed company would like to save on tax part and show lower earnings. Capitalising R&D costs directly leads to addition in assets and hence your profit and loss account are not affected in a single year. The same is amortised over a period of time (around few year) through profit and loss account. It basically means that the company is spreading its R&D costs over few years. However, in pharma it is very difficult to estimate whether R&D can lead to definite sales/profit generation in the future (many projects fail) and a prudent company should expense it directly through profit & loss. I personally feel that until R&D expenditure is related to setting up of new R&D centers (fixed costs), new laboratories or equipments, one should directly expense it from profit and loss account.
There is this nice note from PwC which clearly says, that in generics development, R&D cost shouldn’t be capitalized. But this is as per US GAAP.
https://www.pwc.com/us/en/health-industries/our-perspective/assets/us-gaap-issues-nov-2013.pdf
Background
An entity is developing a generic version of a painkiller that has
been sold in the market by another company for many years.
The technological feasibility of the asset has already been
established because it is a generic version of a product that has
already been approved, and its chemical equivalence has been
demonstrated. The lawyers advising the entity do not anticipate
that any significant difficulties will delay the process of obtaining
commercial regulatory approval.
Relevant guidance
Research and development costs… shall be charged to expense
when incurred [ASC 730–10–25–1].
Solution
No. Research and development costs should be expensed as incurred.
and here some more generic principles followed in capitalizing expense, after reading them, i feel, it’s right on part of generic companies to not capitalize the R&D expense.
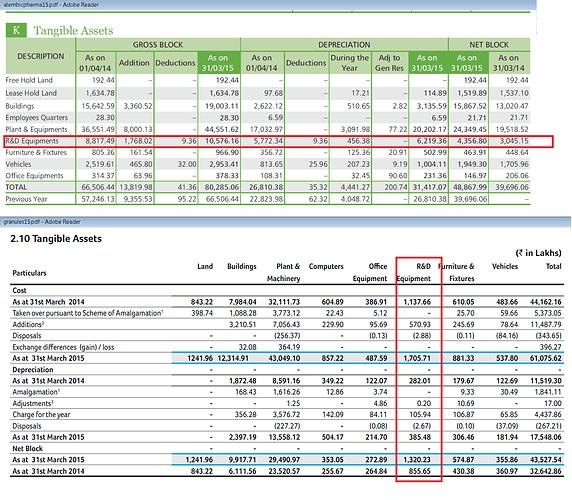
According to Granules and Alembic Annual Report
1…Only R&D Equipment’s are capitalized
2…Other R&D Expenses are shown in P&L
For Granulesheight=“293”> its shown in other Expenses
Alembic under R&D Expenses
<img src="/uploads/default/original/2X/6/64b776e4304d0df87744e61672eed7b7d87b908a.jpg" width=“690”
R&D Expense ie…Capitalized (Only Equipment’s I think it is general practice in India)
R&D Expenses shown in P&L
Alembic Pharma’s Chirayu Amin, family set up offshore firm in 2015, Panama Papers reveal
Vadodara-based Alembic Pharmaceuticals’ promoter Chirayu Amin set up an offshore entity in the British Virgin Islands (BVI) in 2015, a report in The Indian Express has said.
Investigations into the leaked Panama Papers by The Indian Express have revealed that Amin and his family members are listed as the beneficiaries of Whitefield Global Investments Ltd, a company that was incorporated on October 1, 2015. The entity was set up with the sole purpose of buying and renting property in London.
investments seems very small $1 million(6.6 cr) compared to revenues of the company (> 3000 cr ) and into non-competing property investments. moreover, the promoter response was that he followed the law of land. This seems ok to me. Am I missing something here ?
Alembic stock has been falling when the market is reclaiming previous highs.Should that raise alarm and solicit a closer look ? Pharma companies in past have given bad surprises. Could senior boarders suggest how this unusual situation should be approached and what diligence can be performed.?
@ramanhp I empathize with your panic with the sliding stock price of alembic. It seems you haven’t read much of the thread above your post to understand the company’s fundamentals. You seem to be judging the company based on its face value i.e its price in the stock exchange.
Let me attempt to help you a bit. In my understanding, Atleast 10-20% of the price seems to covered up by very short term investors/traders. They just look out for gains in very near future. If you read the recent posts in this thread you will realize two important things:
- The “abilify” effect on the earnings is almost over.
- CFO in recent concall has stated to expend as much as 15% of sales as R&D expenses which would not be capitalized. Thus it would slightly depress the EPS in near term.
These two things are not good news for short term investors. So, one inference from current sliding prices is that the short term investors are selling their shares.
However, the medium to long term view of the company looks intact IMHO.
Hope this helps you.
I would again request you to go through this wonderful thread (atleast last 100 posts).
Disclosure: Invested. Views are biased.
Thanks @nishantkandoi. I have been reading Alembic’s post here and I consider it a Pharma company doing the right things. It was in that context the regular fall in stock price incongruous and hence my question. Agree that the stock price in not always reflective of company’s performance and potential , I just wanted to be sure that I am not missing on any nasty detail.
Hi Raman,
I would refrain from commenting about the price but would give my views. I think it all boils down to your investment horizon. The R&D expenses have increased substantially from Rs.121.62 crore during FY15 to Rs.307.06 crore during FY16. These are expected to further increase to around Rs.450 crore during FY17 as indicated by the management. The number of projects the company is working on has more than tripled over the past one year. This is expected to impact the margins of the company in the near term with the positive effect of gAbilify fading out. The result of the increased R&D spend might not be visible in the near term but will have positive impact over the long term. Also, if one looks at how the company was able to milk the gAbilify opportunity, we can notice that other generic companies like Torrent/Hetero were able to perform better due to their own front end operations. The company had to share a very high proportion of the profits (as high as 50% in some case) made in a drug with its marketing partner. Alembic is now addressing the issue by launching its own front end and most of the new launches will be through its own front end along with taking over the marketing of few of its already approved products from its marketing partner. However, the company might face some teething issues due to launch of its own front end in the near term. Again, it boils down to your investment horizon. The company is venturing into complex segments like oncology, dermatology, injectables etc which bodes well for the future. It has very interesting launches in the near to medium term for the US markets which if receive timely approval from USFDA might help in compensating for the loss of gAbilify. However, even management will not know the timeline for getting approval.
Disclosure: Invested and have more than 10% of my pf in the stock.
Amneal Phams gets approval for gAbilify (Aripiprazole). The link:
http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails
This is the seventh generic player to enter the market. As per the litigation charges by Otsuka, the innovator, there have been more than 20 applications for the generic. However, till date only seven players have got final approval from USFDA. The pricing for the drug has reduced a lot (as per few research reports its more than 80 - 90% of the brand). However, its will still remain a decent opportunity for the generic players given its large market size.